
Concept explainers
(a)
Interpretation:
The structural formula for given alcohol is to be written.
Concept introduction:
The general formula of alcohol is
The alcohols are named by using suffix ‘ol’.
The alcohols are classified as primary, secondary and tertiary on the basis of nature of carbon atom attached to
If carbon containing
If carbon containing
If carbon containing
(a)
Answer to Problem 43A

Explanation of Solution
The prefix ‘ol’ in 1- pentanol shows organic compound contains alcohol
is attached to C-1. The word ‘pentan’ suggest it has five carbon atoms bonded by single bond. So the structural formula is:

1-pentanol is primary alcohol because functional group
(b)
Interpretation:
The structural formula for given alcohol is to be written.
Concept introduction:
The general formula of alcohol is
The alcohols are named by using suffix ‘ol’.
The alcohols are classified as primary, secondary and tertiary on the basis of nature of carbon atom attached to
If carbon containing
If carbon containing
If carbon containing
(b)
Answer to Problem 43A
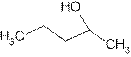
Explanation of Solution
The prefix ‘ol’ in 2- pentanol shows organic compound contains alcohol functional group where
is attached to C-2. The word ‘pentan’ suggest it has five carbon atoms bonded by single bond. So the structural formula is:
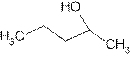
2-pentanol is secondary alcohol because functional group
(c)
Interpretation:
The structural formula for given alcohol is to be written.
Concept introduction:
The general formula of alcohol is
The alcohols are classified as primary, secondary and tertiary on the basis of nature of carbon atom attached to
If carbon containing
If carbon containing
If carbon containing
(c)
Answer to Problem 43A
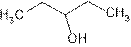
Explanation of Solution
The prefix ‘ol’ in 3- pentanol shows organic compound contains alcohol functional group where
is attached to C-3. The word ‘pentan’ suggest it has five carbon atoms bonded by single bond. So the structural formula is:
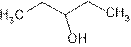
3-pentanol is seconary alcohol because functional group
(d)
Interpretation:
The structural formula for given alcohol is to be written.
Concept introduction:
The general formula of alcohol is
The alcohols are classified as primary, secondary and tertiary on the basis of nature of carbon atom attached to
If carbon containing
If carbon containing
If carbon containing
(d)
Answer to Problem 43A
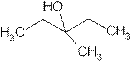
Explanation of Solution
The prefix ‘ol’ in 3-Methyl-3- pentanol shows organic compound contains alcohol functional group where
is attached to C-3. The word ‘pentan’ suggest it has five carbon atoms bonded by single bond. At c-3 one methyl group is attached as a substituent. So the structural formula is:
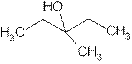
3-Methyl-3- pentanol is tertiary alcohol because functional group
Chapter 20 Solutions
World of Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





