
Study Guide for Campbell Biology
11th Edition
ISBN: 9780134443775
Author: Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Jane B. Reece, Martha R. Taylor, Michael A. Pollock
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 5IQ
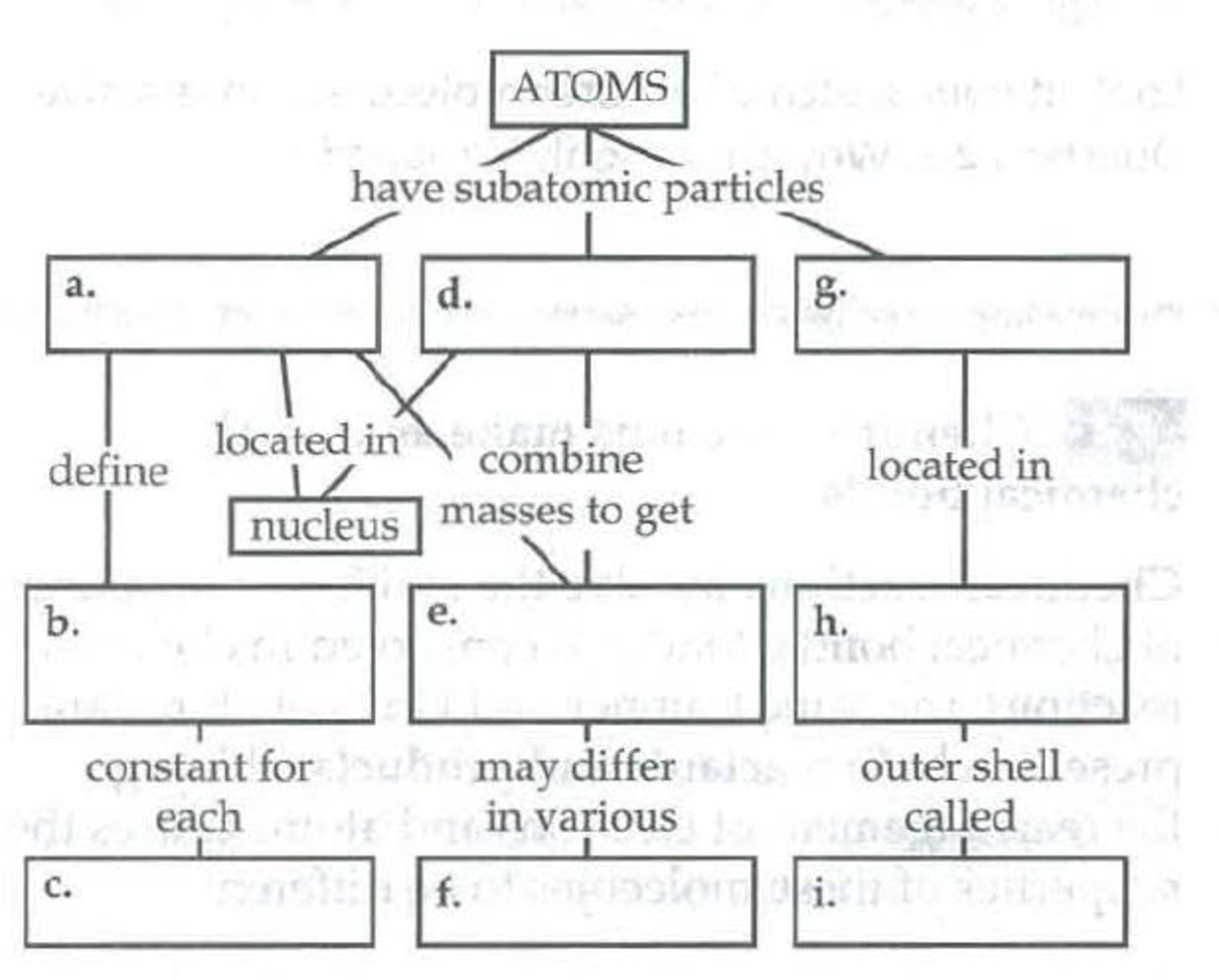
Fill in the blanks in the following concept map to help you review the atomic structure of atoms.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A covalent bond consists of a pair of shared electrons between two atoms. How does this shared pair hold the atoms together? What is the relationship of this covalent bond with Octet rule? Give an example to support your answer.
Explain this statement: “All compounds are molecules, but not allmolecules are compounds.” Give an example.
Draw and label a simplified model of an atom. Explain how this model misrepresents our understanding of atomic structure
Chapter 2 Solutions
Study Guide for Campbell Biology
Ch. 2 - Fill in the names beside the symbols of the...Ch. 2 - The difference between the mass number and the...Ch. 2 - To move to a shell farther from the nucleus, an...Ch. 2 - Prob. 4IQCh. 2 - Fill in the blanks in the following concept map to...Ch. 2 - Prob. 6IQCh. 2 - Prob. 7IQCh. 2 - Prob. 8IQCh. 2 - Draw the structural formula of a water molecule,...Ch. 2 - Look at your sketch of a water molecule in...
Ch. 2 - Prob. 11IQCh. 2 - Prob. 1SYKCh. 2 - Atoms can have various numbers associated with...Ch. 2 - Prob. 3SYKCh. 2 - Prob. 1TYKCh. 2 - Prob. 2TYKCh. 2 - Prob. 3TYKCh. 2 - Prob. 4TYKCh. 2 - Radioactive isotopes can be used in studies of...Ch. 2 - Prob. 6TYKCh. 2 - Prob. 7TYKCh. 2 - Prob. 8TYKCh. 2 - Prob. 9TYKCh. 2 - Prob. 10TYKCh. 2 - Prob. 11TYKCh. 2 - Prob. 12TYKCh. 2 - Prob. 13TYKCh. 2 - Prob. 14TYKCh. 2 - Prob. 15TYKCh. 2 - A covalent bond between two atoms is likely to be...Ch. 2 - Prob. 17TYKCh. 2 - Prob. 18TYKCh. 2 - For questions 19-21, choose from the following...Ch. 2 - Prob. 20TYKCh. 2 - Prob. 21TYKCh. 2 - Prob. 22TYKCh. 2 - Prob. 23TYKCh. 2 - Prob. 24TYKCh. 2 - Prob. 25TYKCh. 2 - What is the difference between a molecule and a...Ch. 2 - Prob. 27TYKCh. 2 - Prob. 28TYK
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- Organic molecules contain both carbon and hydrogen atoms (and may have a few other elements as well). There are millions of kinds of organic molecules. They include molecules like: What is the smallest organic molecule?arrow_forwardThe “octet rule” in chemistry helps predict the types of bonds thatatoms will form. In general, an atom will be most stable if it fills itsouter shell of 8 electrons. Atoms with fewer than 4 valence electronstend to donate electrons and those with more than 4 valence electronstend to accept additional electrons; those with exactly 4 can do both.Using this rule, determine what category each of the followingelements falls into: N, S, C, P, O, H, Ca, Fe, and Mg. (You will needto work out the valence of the atoms.)arrow_forwardfill the following table polymer or large biological molecule monomer or smaller subunit one funtion name od covalent bond nulceic acids three fatty acids easter bond acts as an enzyme immediate or long-term energy source glycosidic linkage explain the chemical reaction that occurs water molecules dissociate and reform. why is these chemical reactions the basis of the pH scale?arrow_forward
- Using the image below answer the following question. What does "3" indicate? 2 - CH2 – CH2 – CH2 – CH2 – NH,* O-ċ- CH,- CH2 3 C- NH2 ČH 4 CH2 H3C CH3 H3C CH; CH o a. Hydrophobic interaction o b. Disulfide linkage O. Polypeptide backbone od. lonic bond o e. Hydrogen bondarrow_forwardKnowing the atomic mass of an element allows inferences about which of the following? the number of protons plus neutrons in the element the number of protons plus electrons in the element the number of electrons in the element the number of protons in the elementarrow_forwardNitrogen is an isotope of nitrogen that has 7 protons and 3 neutrons.What are the atomic atom and atomic mass number of nitrogen 11 here is the picture here.arrow_forward
- Fill in the following study table. You might make a bigger version if you need more room. Type of biological molecule Monomer Examples and their functions Other facts or interesting information Carbohydrate Lipid Protein Nucleic Acidarrow_forwardCovalent and ionic bonds are the main features of nearly every biological molecule. Discuss how each of these bonds forms, and why they are so important in biological molecules.arrow_forwardAtoms A and B interact to form a compound, AB2. When measured, atom A has a partial negative charge and atom B has a partial positive charge. From this information, we can conclude what? Select only ONE answer choice. Note: - means "approximately equal to" , A > B means "A is greater than B" , and A B: AB2 is hydrophilic Not enough information to answer the questionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning

Concepts of Biology
Biology
ISBN:9781938168116
Author:Samantha Fowler, Rebecca Roush, James Wise
Publisher:OpenStax College

Biology Today and Tomorrow without Physiology (Mi...
Biology
ISBN:9781305117396
Author:Cecie Starr, Christine Evers, Lisa Starr
Publisher:Cengage Learning
Macromolecules | Classes and Functions; Author: 2 Minute Classroom;https://www.youtube.com/watch?v=V5hhrDFo8Vk;License: Standard youtube license