
a)
Interpretation : The pH at the end point of this titration is to be calculated.
Concept Introduction : The negative logarithm of the concentration of hydrogen ions is the pH of a solution. The formula to calculate pH is given as
a)
Answer to Problem 93A
The pH at the end point is
Explanation of Solution
The concentration of
The volume of ethanoic acid is
The reaction taking place between sodium hydroxide and ethanoic acid is given as
Since the molar ratio is 1:1.
To neutralize the 50 ml of ethanoic acid, there is a need of 50 ml of sodium hydroxide.
The endpoint is reached when 50 ml of sodium hydroxide is added.
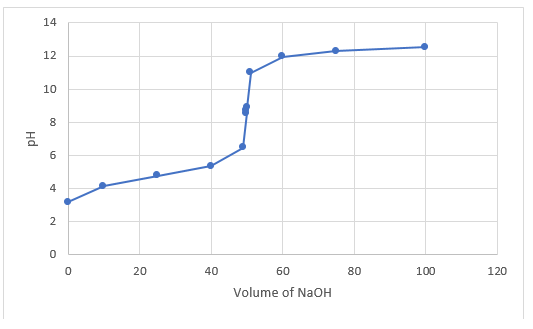
With the help of data, pH at 50 ml of sodium hydroxide is detected.
So, the pH at the end point is 
The graph is plotted between volume of sodium hydroxide and pH.
b)
Interpretation : The acid-base indicators that could be used to determine the end point in this titration is to be identified.
Concept Introduction : An indicator is any substance that alters color to signal the presence or absence of a chemical species in a solution, such as an acid or an alkali.
b)
Answer to Problem 93A
Phenolphthalein or phenol red can be used as indicators for
Explanation of Solution
An indicator is any substance that alters color to signal the presence or absence of a chemical species in a solution, such as an acid or an alkali.
The titration's end occurs when the indicator's color changes.
For acid-base titration, the end point is detected by indicators like phenolphthalein or phenol red.
Chapter 19 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





