
Concept explainers
Interpretation: The correct sterochemical descriptor for the substitution pattern of the cyclopropane ring in permethrin and bifentrin has to be determined.
Concept introduction:
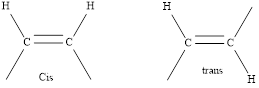
Cis compounds are the geometrical isomers with the organic groups on the same side of the double bond.
The double bond of
In a cis isomer, groups are attached on the same side of the double bond while in the Trans isomer the groups are attached on the opposite sides.
Simple representation of cis and Trans isomer is as follows,
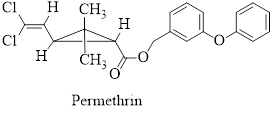
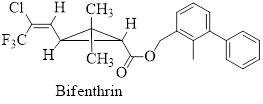
Permethrin and bifenthrin are the synthetic pyretherenoids now in common use in household and agricultural products.
Their structures are given below,
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
Organic Chemistry, Loose-leaf Version
- Ethyl butyrate, CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring.It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l). The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a 78.0% yield. How many grams would be produced from 8.50 gof butanoic acid and excess ethanol? Express your answer in grams to three significant figures.arrow_forwardEthyl butyrate, CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring.It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l) Given 8.50 g of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100%yield? Express your answer in grams to three significant figures.arrow_forwardDigitalis is a preparation made from the dried seeds and leaves of the purple foxglove, Digitalis purpurea, a plant native to southern and central Europe and cultivated in the United States. The preparation is a mixture of several active components, including digitalin. Digitalis is used in medicine to increase the force of myocardial contraction and as a conduction depressant to decrease heart rate (the heart pumps more forcefully but less often).arrow_forward
- Lipoic acid is required by many microorganisms for proper growth. As a disulfide, it functions in the living system by catalyzing certain oxidation reactions and is reduced in the process. Write the structure of the reduction product.arrow_forwardPermethrin and Bifenthrin Pyrethrin is a natural insecticide obtained from the powdered flower heads of several species of Chrysanthemum. The active substances in pyrethrum, principally pyrethrins I and II, are contact poisons for insects and cold-blooded vertebrates. Although powders made from Chrysanthemum extracts have found widespread use, the active substances in them are destroyed rapidly in the environment. In an effort to develop synthetic compounds as effective as the natural insecticides but with greater biostability, chemists prepared a series of esters related in structure. Among the synthetic pyrethrenoids now in common use in household and agricultural products are permethrin and bifenthrin. As discussed above, the natural products pyrethrins I and II (not shown) are destroyed rapidly in the environment. One of the key changes in the structures of permethrin and bifenthrin relative to the pyrethrins was the substitution of naturally occurring methyl groups on the alkene with electron withdrawing groups (EWGs) such as chlorine and trifluoromethyl. What reactions of the alkene would the change of methyl groups to EWGs retard? 1. Oxidation of the double bond. 2. Electrophilic addition reactions. 3. Nucleophilic addition reactions. 4. Both 1 and 2. 5. All of the above.arrow_forwardVanilla flavoring is either extracted from a tropical orchid or synthetically produced from wood pulp by-products. What differences in chemical structure would you expect in these two commercial products: vanilla extract and imitation vanilla extract?arrow_forward
- Account for the fact that treating propenoic acid (acrylic acid) with HCl gives only 3-chloropropanoic acid.arrow_forwardSuggest a possible structure for Compound X.arrow_forwardIn the mid-1930s a substance was isolated from a fungus that is a parasite of ryes and other grasses. This alkaloid, lysergic acid, has been of great interest to chemists because of its strange, dramatic action on the human mind. Many derivatives of lysergic acid are known, some with medicinal applications. Perhaps the best known derivative of lysergic acid is the potent hallucinogen lysergic acid diethylamide (LSD): మగవా జి N-H LSD (CH25N;O) Like other alkaloids, LSD is a weak base, with Kp = 7.6 × 107. What is the pH of a 0.94 M solution of LSD? pH =arrow_forward
- Salsalate, which is an ester formed by the reaction of two molecules of salicylic acid, is another salicylate used as an aspirin alternative for those who are hypersensitive to aspirin. Draw the structures of salicylic acid and salsalate.arrow_forwardIn early 1960s, the National Cancer Institute was able to isolate a complex organic compound from the bark of the Pacific Yew, Taxus brevifolia. The structure of the cancer-fighting component of yew bark was determined in 1962, and the compound was names paclitaxel (Taxol). Taxol is an anticancer agent active against ovarian, breast, and some lung tumors. Given the structure of Taxol, identify and place an asterisk at the stereogenic or chirality centers present within its complex structures. (Hint: There are only 11 chirality centers). ΝΗ O OH Paclitaxel (Taxol) ill HO H OHarrow_forwardExplain the solubility behavior of aniline, diethylamine and N,N-diethylaniline in water.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co




