
Organic Chemistry, Loose-leaf Version
8th Edition
ISBN: 9781305865549
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 17.8, Problem 17.5P
(a)
Interpretation Introduction
Interpretation: The given reaction equation has to completed.
Concept introduction:
Acid chlorides are organic compound and they have
Acid chlorides are most often prepared by treating a
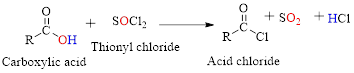
(b)
Interpretation Introduction
Interpretation: The given reaction equation has to completed.
Concept introduction:
Acid chlorides are organic compound and they have functional group
Acid chlorides are most often prepared by treating a carboxylic acid with thionyl chloride.
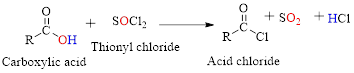
The
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Write the structural formulas of trichloroacetic acid and acetic acid and explain why trichloroacetic acid is the much stronger acid of the two.
The color dye that is positively charged.a) Eosin Red c) Phenol Redb) Bromcresol Green d) Gentian Violet
Ester compounds often have a sweet, pleasant odor. Many
characteristic fruit scents are largely due to the natural presence
of one or more ester compounds. As such, artificial scents for
foods are often composed of complex mixtures of various esters.
The exact identity and ratio of ingredients that compose a
particular scent are closely guarded secrets in the food and
fragrance industry.
Suppose that you are a chemist working for a company that i
creating a new line of air fresheners. The company is considering
three scents: apple, pear, and pineapple. The project manager has
asked you to prepare the ester compounds that are largely
responsible for these scents. The structural formulas for these
ester compounds are shown here:
Alcohols for Air Freshener Project
Molar mass Density Cost, per
(g/mL)
Reagent
(g/mol)
1.00 L
methanol
32.04
0.79
$46.20
ethanol
46.07
0.79
$112.00
1-propanol
60.10
0.80
$72.70
1-butanol
74.12
0.81
$72.60
Use the structural formulas of the alcohols and…
Chapter 17 Solutions
Organic Chemistry, Loose-leaf Version
Ch. 17.2 - Prob. 17.1PCh. 17.4 - Which is the stronger acid in each pair?Ch. 17.4 - Prob. 17.3PCh. 17.7 - Prob. 17.4PCh. 17.8 - Prob. 17.5PCh. 17.8 - Prob. AQCh. 17.8 - Prob. BQCh. 17.8 - Prob. CQCh. 17.8 - Permethrin and Bifenthrin Pyrethrin is a natural...Ch. 17.9 - Prob. 17.6P
Ch. 17 - Write the IUPAC name of each compound, showing...Ch. 17 - Prob. 17.8PCh. 17 - Prob. 17.9PCh. 17 - Prob. 17.10PCh. 17 - Prob. 17.11PCh. 17 - Prob. 17.12PCh. 17 - Prob. 17.13PCh. 17 - On a cyclohexane ring, an axial carboxyl group has...Ch. 17 - Prob. 17.15PCh. 17 - Prob. 17.16PCh. 17 - Prob. 17.17PCh. 17 - Complete each reaction.Ch. 17 - Prob. 17.19PCh. 17 - Prob. 17.20PCh. 17 - Prob. 17.21PCh. 17 - Show the reagents and experimental conditions...Ch. 17 - Prob. 17.23PCh. 17 - Prob. 17.24PCh. 17 - Prob. 17.25PCh. 17 - In each set, assign the acid its appropriate pKa.Ch. 17 - Low-molecular-weight dicarboxylic acids normally...Ch. 17 - Complete the following acid-base reactions. (a)...Ch. 17 - Prob. 17.29PCh. 17 - Prob. 17.30PCh. 17 - Excess ascorbic acid is excreted in the urine, the...Ch. 17 - Give the expected organic product when...Ch. 17 - Show how to convert trans-3-phenyl-2-propenoic...Ch. 17 - Show how to convert 3-oxobutanoic acid...Ch. 17 - Prob. 17.35PCh. 17 - Prob. 17.36PCh. 17 - Prob. 17.37PCh. 17 - When 4-hydroxybutanoic acid is treated with an...Ch. 17 - Fischer esterification cannot be used to prepare...Ch. 17 - Draw the product formed on thermal decarboxylation...Ch. 17 - Prob. 17.41PCh. 17 - Show how cyclohexanecarboxylic acid could be...Ch. 17 - Prob. 17.43PCh. 17 - Prob. 17.44PCh. 17 - Prob. 17.45PCh. 17 - Write the products of the following sequences of...Ch. 17 - Using your reaction roadmaps as a guide, show how...Ch. 17 - Using your reaction roadmaps as a guide, show how...Ch. 17 - Using your reaction roadmaps as a guide, show how...Ch. 17 - Using your reaction roadmaps as a guide, show how...Ch. 17 - Prob. 17.51PCh. 17 - Complete the following Fischer esterification...Ch. 17 - Prob. 17.53P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Lipoic acid is required by many microorganisms for proper growth. As a disulfide, it functions in the living system by catalyzing certain oxidation reactions and is reduced in the process. Write the structure of the reduction product.arrow_forwardComplete the following reactions: a. b.arrow_forward3. What happen during redox, dehydration, hydration and decarboxylic reaction? Give examples of each of the reaction.arrow_forward
- Complete the following reactions: a. b. c.CH3SSCH2CH3+2(H)arrow_forwardGive IUPAC names for the following substances:arrow_forwardWrite equations to show how the following conversions can be achieved. More than one reaction is required, and reactions from earlier chapters may be necessary. a. CH3CH=CHCH3CH3COCH2CH3 b. CH3CH2CH2OHCH3CH2COOHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY