
(a)
Interpretation: The name of
Concept introduction:
Esters encompass a large family of organic compound with broad application in various fields of science and technology.
In Fischer esterification, a carboxylic acid is reacted with an alcohol in the presence of an acid catalyst, such as concentrated sulfuroc acid.
Esters can be simply represented as,
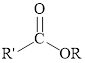
Fischer esterification can be represented as follows,
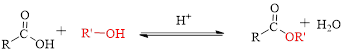
The splitting of an ester into the component acid and alcohol is known as ester hydrolysis.
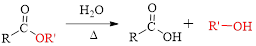
(b)
Interpretation: The name of carboxylic acid and alcohol has to be determined, from which the given eseter is derived.
Concept introduction:
Esters encompass a large family of organic compound with broad application in various fields of science and technology.
In Fischer esterification, a carboxylic acid is reacted with an alcohol in the presence of an acid catalyst, such as concentrated sulfuroc acid.
Esters can be simply represented as,

Fischer esterification can be represented as follows,
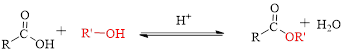
The splitting of an ester into the component acid and alcohol is known as ester hydrolysis.
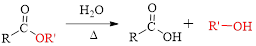
(c)
Interpretation: The name of carboxylic acid and alcohol has to be determined, from which the given eseter is derived.
Concept introduction:
Esters encompass a large family of organic compound with broad application in various fields of science and technology.
In Fischer esterification, a carboxylic acid is reacted with an alcohol in the presence of an acid catalyst, such as concentrated sulfuroc acid.
Esters can be simply represented as,

Fischer esterification can be represented as follows,
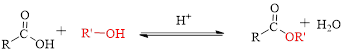
The splitting of an ester into the component acid and alcohol is known as ester hydrolysis.
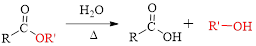
(d)
Interpretation: The name of carboxylic acid and alcohol has to be determined, from which the given eseter is derived.
Concept introduction:
Esters encompass a large family of organic compound with broad application in various fields of science and technology.
In Fischer esterification, a carboxylic acid is reacted with an alcohol in the presence of an acid catalyst, such as concentrated sulfuroc acid.
Esters can be simply represented as,

Fischer esterification can be represented as follows,
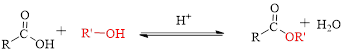
The splitting of an ester into the component acid and alcohol is known as ester hydrolysis.
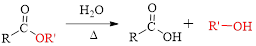
Trending nowThis is a popular solution!

Chapter 17 Solutions
Organic Chemistry, Loose-leaf Version
- 1. What are the common derivatives of carboxylic acids? How are they named? 2. What reactions do carboxylic acid derivatives undergo?arrow_forwardWhich isomer category represents the relationship between carboxylic acid and ester?arrow_forwardWrite the two step reaction that would produce benzoic acid from benzenearrow_forward
- Ester compounds often have a sweet, pleasant odor. Many characteristic fruit scents are largely due to the natural presence of one or more ester compounds. As such, artificial scents for foods are often composed of complex mixtures of various esters. The exact identity and ratio of ingredients that compose a particular scent are closely guarded secrets in the food and fragrance industry. Suppose that you are a chemist working for a company that i creating a new line of air fresheners. The company is considering three scents: apple, pear, and pineapple. The project manager has asked you to prepare the ester compounds that are largely responsible for these scents. The structural formulas for these ester compounds are shown here: Alcohols for Air Freshener Project Molar mass Density Cost, per (g/mL) Reagent (g/mol) 1.00 L methanol 32.04 0.79 $46.20 ethanol 46.07 0.79 $112.00 1-propanol 60.10 0.80 $72.70 1-butanol 74.12 0.81 $72.60 Use the structural formulas of the alcohols and…arrow_forwardOne of the compounds that gives orange oil its unique odor is an ester formed when acetic acid reacts with octan-1-ol. Draw the structure of this ester and name it.arrow_forwardExplain the characteristic reaction of aldehydes and ketones ?arrow_forward
- Write a general equation showing the preparation of a carboxylic acid from an alcohol.arrow_forwardDraw the structure for the carboxylic acid formed by the mild oxidation of 2-propanone.arrow_forwardDescribe the acidity of different carboxylic acids and predict the products obtained when they react with strong bases.arrow_forward
- what are similarities and differences between Ketones,Aldehydes,and Carboxylic acidsarrow_forwardExplain the reaction of aldehydes and ketones with nitrogen ?arrow_forwardWhat functional group would be present in the product (the main organic product) of a reaction between an alcohol and a carboxylic acid with an acid catalyst?arrow_forward

 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





