
Concept explainers
Practice Problem 17.9
Esters can also be synthesized by transesterification:
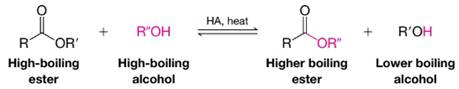
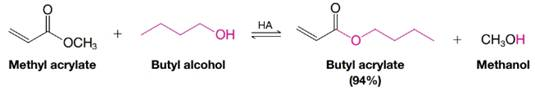
In this procedure we shift the equilibrium to the right by allowing the low-boiling alcohol to distill from the reaction mixture. The mechanism for transesterification is similar to that for an acid-catalyzed esterification (or an acid-catalyzed ester hydrolysis). Write a detailed mechanism for the following transesterification:
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Fundamentals of Anatomy & Physiology (11th Edition)
Principles of Anatomy and Physiology
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Cosmic Perspective Fundamentals
- this is an organic chemistry question please answer accordindly!! please post the solution draw the figures on a paper please hand drawn and post, please answer EACH part till the end and dont just provide wordy explanations, please draw them on a paper and post clearly!! answer the full question with all details EACH PART CLEARLY please thanks!! im reposting this please solve all parts and draw it not just word explanations!!arrow_forwardA mixture of 0.412 M C12, 0.544 M F2, and 0.843 M CIF is enclosed in a vessel and heated to 2500 K. C12(g) + F2(g )2CIF(g) Kc = 20.0 at 2500 K Calculate the equilibrium concentration of each gas at 2500 K. [C12] = M [F2] = M [ CIF] =arrow_forwardShow reaction mechanism with explanation. don't give Ai generated solutionarrow_forward
- Don't used Ai solutionarrow_forwardthis is an organic chemistry question please answer accordindly!! please post the solution draw the figures and post, answer the question in a very simple and straight forward manner thanks!!!!! please answer EACH part till the end and dont just provide wordy explanations wherever asked for structures or diagrams, please draw them on a paper and post clearly!! answer the full question with all details EACH PART CLEARLY please thanks!! im reposting this kindly solve all parts and draw it not just word explanations!!arrow_forwardPlease correct answer and don't used hand raitingarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT



