
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 17, Problem 16PP
Practice Problem 17.16
Diacyl peroxides, 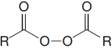 , decompose readily when heated.
, decompose readily when heated.
(a) What factor accounts for this instability?
(b) The decomposition of a diacyl peroxide produces
(c) Diacyl peroxides are often used to initiate radical reactions, such as in the
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Show work. don't give Ai generated solution
Don't used Ai solution
Don't used Ai solution
Chapter 17 Solutions
Organic Chemistry
Ch. 17 - Practice Problem 17.1 Give an IUPAC systematic...Ch. 17 - Prob. 2PPCh. 17 - Practice Problem 17.3 Write structural formulas...Ch. 17 - Practice Problem 17.4
Show how each of the...Ch. 17 - Practice Problem 17.5
Show how you could prepare...Ch. 17 - Practice Problem 17.6
(a) Which of the carboxylic...Ch. 17 - Prob. 7PPCh. 17 - Prob. 8PPCh. 17 - Practice Problem 17.9
Esters can also be...Ch. 17 - Prob. 10PP
Ch. 17 - Prob. 11PPCh. 17 - Practice Problem 17.12
What products would you...Ch. 17 - Practice Problem 17.13 (a) Provide the reagents...Ch. 17 - Prob. 14PPCh. 17 - Practice Problem 17.15 Using decarboxylation...Ch. 17 - Practice Problem 17.16 Diacyl peroxides, ,...Ch. 17 - Prob. 17PCh. 17 - Give an IUPAC systematic or common name for each...Ch. 17 - Prob. 19PCh. 17 - Prob. 20PCh. 17 - 17.21 What major organic product would you expect...Ch. 17 - Prob. 22PCh. 17 - Prob. 23PCh. 17 - Prob. 24PCh. 17 - Prob. 25PCh. 17 - 17.26 What products would you expect to obtain...Ch. 17 - Write structural formulas for the major organic...Ch. 17 - 17.28 Indicate reagents that would accomplish each...Ch. 17 - Write structural formulas for the major organic...Ch. 17 - Prob. 30PCh. 17 - Prob. 31PCh. 17 - Prob. 32PCh. 17 - 17.33 On heating,...Ch. 17 - Prob. 34PCh. 17 - Prob. 35PCh. 17 - 17.36 Show how pentanoic acid can be prepared from...Ch. 17 - 17.37 The active ingredient of the insect...Ch. 17 - Prob. 38PCh. 17 - Prob. 39PCh. 17 - Give stereochemical formulas for compounds AQ:...Ch. 17 - 17.41 -Glyceraldehyde can be transformed into...Ch. 17 - Prob. 42PCh. 17 - Prob. 43PCh. 17 - 17.44 Given here are the NMR spectra and carbonyl...Ch. 17 - 17.45 Compound Y dissolves slowly when warmed...Ch. 17 - Prob. 46PCh. 17 - Prob. 47PCh. 17 - Prob. 48PCh. 17 - Prob. 49PCh. 17 - Prob. 50PCh. 17 - Prob. 51PCh. 17 - 17.52 Starting with 1-naphthol, suggest an...Ch. 17 - Suggest a synthesis of ibuprofen (Section 5.11)...Ch. 17 - Prob. 54PCh. 17 - Prob. 55PCh. 17 - Prob. 1LGPCh. 17 - Prob. 2LGPCh. 17 - Prob. 3LGPCh. 17 - Prob. 4LGP
Additional Science Textbook Solutions
Find more solutions based on key concepts
Sketch the following spectra that would be obtained for 2-chloroethanol: a. The 1H NMR spectrum for an anhydrou...
Organic Chemistry (8th Edition)
44. Calculate the ratio of CH3NH2 to CH3NH3Cl concentration required to create a buffer with pH = 10.24.
Chemistry: A Molecular Approach (4th Edition)
How do food chains and food webs differ? Which is the more accurate representation of feeding relationships in ...
Biology: Life on Earth (11th Edition)
Why are the top predators in food chains most severely affected by pesticides such as DDT?
Campbell Essential Biology (7th Edition)
8.63 Two flasks of equal volume and at the same temperature contain different gases. One flask contains 10.0 g ...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Match each of the following items with all the terms it applies to:
Human Physiology: An Integrated Approach (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Ph heat heatarrow_forward(12) Which one of the following statements about fluo- rometry is FALSE? a) Fluorescence is better detected at 90 from the exci- tation direction. b) Fluorescence is typically shifted to longer wave- length from the excitation wavelength. c) For most fluorescent compounds, radiation is pro- duced by a transitionarrow_forwardDon't used Ai solutionarrow_forward
- (f) SO: Best Lewis Structure 3 e group geometry:_ shape/molecular geometry:, (g) CF2CF2 Best Lewis Structure polarity: e group arrangement:_ shape/molecular geometry: (h) (NH4)2SO4 Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forward1. Problem Set 3b Chem 141 For each of the following compounds draw the BEST Lewis Structure then sketch the molecule (showing bond angles). Identify (i) electron group geometry (ii) shape around EACH central atom (iii) whether the molecule is polar or non-polar (iv) (a) SeF4 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: (b) AsOBr3 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles):arrow_forward(c) SOCI Best Lewis Structure 2 e group arrangement: shape/molecular geometry:_ (d) PCls Best Lewis Structure polarity: e group geometry:_ shape/molecular geometry:_ (e) Ba(BrO2): Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY