
Organic Chemistry
4th Edition
ISBN: 9780073402772
Author: Janice G. Smith
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 16.18P
Draw the product formed when each diene and dienophile react in a Diels–Alder reaction.
a. 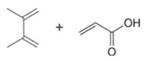 b.
b. 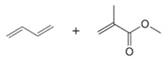 c.
c. 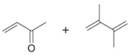
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
reaction scheme for C39H4202
Hydrogenation of Alkyne (Alkyne to
Alkene)
show reaction (drawing) please
Give detailed mechanism Solution with explanation needed. Don't give Ai generated solution
Show work with explanation needed....don't give Ai generated solution
Chapter 16 Solutions
Organic Chemistry
Ch. 16 - Prob. 16.1PCh. 16 - Prob. 16.2PCh. 16 - Draw a second resonance structure for each...Ch. 16 - Prob. 16.4PCh. 16 - Prob. 16.5PCh. 16 - Prob. 16.6PCh. 16 - Prob. 16.7PCh. 16 - Determine the hybridization of the labeled atom in...Ch. 16 - Problem 16.10 Draw the structure consistent with...Ch. 16 - Problem 16.11 Neuroprotectin D1 (NPD1) is...
Ch. 16 - Problem 16.12 Using hybridization, predict how the...Ch. 16 - Problem 16.13 Use resonance theory to explain why...Ch. 16 - Prob. 16.13PCh. 16 - Prob. 16.14PCh. 16 - Prob. 16.15PCh. 16 - Problem 16.17 Draw a stepwise mechanism for the...Ch. 16 - Prob. 16.17PCh. 16 - Problem 16.19 Draw the product formed when each...Ch. 16 - Prob. 16.19PCh. 16 - Prob. 16.20PCh. 16 - Rank the following dienophiles in order of...Ch. 16 - Prob. 16.22PCh. 16 - Prob. 16.23PCh. 16 - Prob. 16.24PCh. 16 - Prob. 16.25PCh. 16 - Problem 16.27 Which compound in each pair absorbs...Ch. 16 - Prob. 16.27PCh. 16 - 16.29 Name each diene and state whether the...Ch. 16 - Prob. 16.29PCh. 16 - Prob. 16.30PCh. 16 - Prob. 16.31PCh. 16 - Prob. 16.32PCh. 16 - Prob. 16.33PCh. 16 - Prob. 16.34PCh. 16 - 16.35 Explain why the cyclopentadienide anion A...Ch. 16 - Explain each statement using resonance theory. a....Ch. 16 - 16.37 Draw the structure of each compound.
a. in...Ch. 16 - Draw and name all dienes of molecular formula...Ch. 16 - Prob. 16.39PCh. 16 - 16.39 Label each pair of compounds as...Ch. 16 - Prob. 16.41PCh. 16 - 16.41 Draw the products formed when each compound...Ch. 16 - Prob. 16.43PCh. 16 - 16.43 Treatment of alkenes A and B with gives the...Ch. 16 - 16.44 Draw a stepwise mechanism for the following...Ch. 16 - Prob. 16.46PCh. 16 - 16.46 Explain, with reference to the mechanism,...Ch. 16 - Prob. 16.48PCh. 16 - Prob. 16.49PCh. 16 - Prob. 16.50PCh. 16 - Prob. 16.51PCh. 16 - Prob. 16.52PCh. 16 - Prob. 16.53PCh. 16 - 16.53 Diels–Alder reaction of a monosubstituted...Ch. 16 - Prob. 16.55PCh. 16 - Prob. 16.56PCh. 16 - 16.55 Devise a stepwise synthesis of each compound...Ch. 16 - Prob. 16.58PCh. 16 - 16.57 A transannular Diels–Alder reaction is an...Ch. 16 - Draw a stepwise mechanism for the following...Ch. 16 - Draw the products of each reaction. Indicate the...Ch. 16 - Prob. 16.62PCh. 16 - Prob. 16.63PCh. 16 - Prob. 16.64PCh. 16 - 16.65 The treatment of isoprene with one...Ch. 16 - 16.66 The treatment of with forms B (molecular...Ch. 16 - Rank the following compounds in the order of...Ch. 16 - Prob. 16.68PCh. 16 - Prob. 16.69PCh. 16 - Prob. 16.70PCh. 16 - Prob. 16.71PCh. 16 - Prob. 16.72PCh. 16 - Prob. 16.73PCh. 16 - Prob. 16.74P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Pleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardThe Ksp for lead iodide ( Pbl₂) is 1.4 × 10-8. Calculate the solubility of lead iodide in each of the following. a. water Solubility = mol/L b. 0.17 M Pb(NO3)2 Solubility = c. 0.017 M NaI mol/L Solubility = mol/Larrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY