
Concept explainers
(a)
Interpretation:
To draw all reasonable resonance structures for the given species
Concept introduction:
Resonance structure is an important concept in
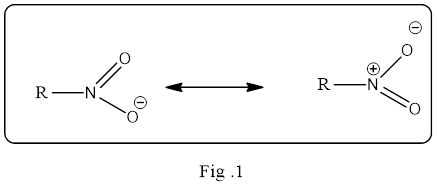
The resonance structure must be shown in double headed resonance arrow to separate them. Example for the resonance structure is given below.
(b)
Interpretation:
To draw all reasonable resonance structures for the given species
Concept introduction:
Resonance structure is an important concept in organic chemistry and it must be understand what it means exactly. The resonance structure does not mean the structure is flipping back and forth between the two structures. It is a static and an average of the two resonance structure. Simply, it is a mixture of all possible resonance structure. And it is the electron pairs drawn in different location in the structure that are delocalized. If the delocalization of electron density is in large volume then the resonance hybrid structure is a stable one.
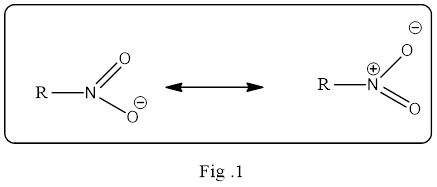
The resonance structure must be shown in double headed resonance arrow to separate them. Example for the resonance structure is given below.
(c)
Interpretation:
To draw all reasonable resonance structures for the given species
Concept introduction:
Resonance structure is an important concept in organic chemistry and it must be understand what it means exactly. The resonance structure does not mean the structure is flipping back and forth between the two structures. It is a static and an average of the two resonance structure. Simply, it is a mixture of all possible resonance structure. And it is the electron pairs drawn in different location in the structure that are delocalized. If the delocalization of electron density is in large volume then the resonance hybrid structure is a stable one.
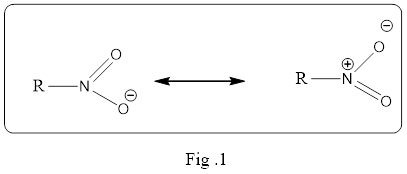
The resonance structure must be shown in double headed resonance arrow to separate them. Example for the resonance structure is given below.
(d)
Interpretation:
To draw all reasonable resonance structures for the given species
Concept introduction:
Resonance structure is an important concept in organic chemistry and it must be understand what it means exactly. The resonance structure does not mean the structure is flipping back and forth between the two structures. It is a static and an average of the two resonance structure. Simply, it is a mixture of all possible resonance structure. And it is the electron pairs drawn in different location in the structure that are delocalized. If the delocalization of electron density is in large volume then the resonance hybrid structure is a stable one.
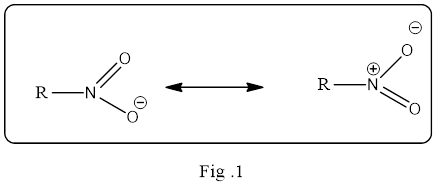
The resonance structure must be shown in double headed resonance arrow to separate them. Example for the resonance structure is given below.
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
Organic Chemistry
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Select to Edit Arrows H H Select to Add Arrows > H CFCI: Select to Edit Arrows H Select to Edit Arrowsarrow_forwardShow work with explanation needed. don't give Ai generated solutionarrow_forwardShow work. don't give Ai generated solutionarrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning


