
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 31P
Assign structures to each of the compounds A, B, and C whose
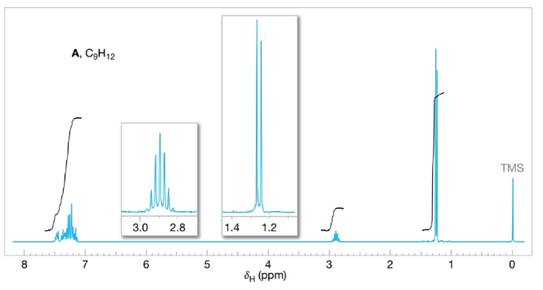
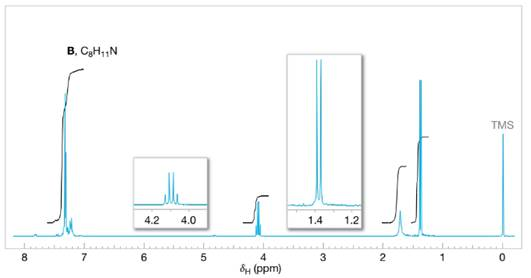
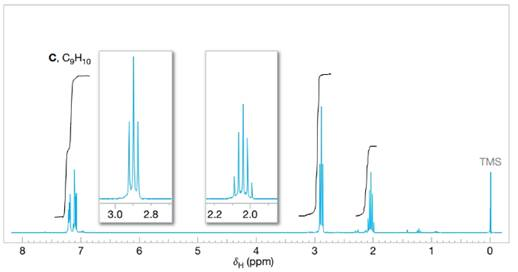
FIGURE 14.27 The
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Assign the H NMR spectra of compounds 1 and 2 and discuss their main differences and similarities. Use the numbering system 1-5 provided in the chemical structure of compound 1 and 6-12 for compound 2. And labels A-E and F-L provided for resonance signal peaks in spectra
1. How many different sets of equivalent aromatic (benzene ring) carbons can be seen in the 13C NMR spectrum of your unknown monosubstituted benzene starting material?
A. Are there any peaks for non-aromatic carbons in the 13C NMR spectrum of your unknown monosubstituted benzene starting material (yes or no)?
B. If yes for part A, please list the corresponding ppm value(s) for any non-aromatic carbon(s)?
Predict how many peaks you would expect to observe in the ‘H NMR,spectrum of the compound shown below and indicate the integration value you would expect to see for each peak. Please explain how you got the answer. (It may be useful to label each hydrogen atom using lowercase letters).
Chapter 14 Solutions
Organic Chemistry
Ch. 14 - PRACTICE PROBLEM 14.1 Provide a name for each of...Ch. 14 - Prob. 2PPCh. 14 - Prob. 3PPCh. 14 - Practice Problem 14.4 Apply the polygon-and-circle...Ch. 14 - Practice Problem 14.5 Apply the polygon-and-circle...Ch. 14 - Practice Problem 14.6 1,3,5-Cycloheptatriene is...Ch. 14 - Prob. 7PPCh. 14 - Prob. 8PPCh. 14 - Practice Problem 14.9 In 1967 R. Breslow (of...Ch. 14 - Prob. 10PP
Ch. 14 - Practice Problem 14.11 In addition to a signal...Ch. 14 - PRACTICE PROBLEM 14.12
Azulene has an appreciable...Ch. 14 - Practice Problem 14.13 (a) The -Sh group is...Ch. 14 - Practice Problem 14.14
Explain how NMR...Ch. 14 - PRACTICE PROBLEM 14.15 Four benzenoid compounds,...Ch. 14 - Prob. 16PCh. 14 - Write structural formulas and give acceptable...Ch. 14 - Prob. 18PCh. 14 - Prob. 19PCh. 14 - Prob. 20PCh. 14 - Which of the hydrogen atoms shown below is more...Ch. 14 - 14.22 The rings below are joined by a double bond...Ch. 14 - Prob. 23PCh. 14 - 14.24 (a) In 1960 T. Katz (Columbia University)...Ch. 14 - Prob. 25PCh. 14 - Prob. 26PCh. 14 - 14.27 5-Chloro-1,3-cyclopentadiene (below)...Ch. 14 - Prob. 28PCh. 14 - Furan possesses less aromatic character than...Ch. 14 - 14.30 For each of the pairs below, predict...Ch. 14 - Assign structures to each of the compounds A, B,...Ch. 14 - Prob. 32PCh. 14 - Give a structure for compound F that is consistent...Ch. 14 - Prob. 34PCh. 14 - Prob. 35PCh. 14 - The IR and 1H NMR spectra for compound X(C8H10)...Ch. 14 - Prob. 37PCh. 14 - Prob. 38PCh. 14 - 14.39 Given the following information, predict the...Ch. 14 - Consider these reactions: The intermediate A is a...Ch. 14 - Prob. 41PCh. 14 - Compound E has the spectral features given below....Ch. 14 - Draw all of the molecular orbitals for...Ch. 14 - Prob. 1LGPCh. 14 - Prob. 2LGPCh. 14 - 3. The NMR signals for the aromatic hydrogens of...Ch. 14 - Prob. 4LGPCh. 14 - Prob. 5LGP
Additional Science Textbook Solutions
Find more solutions based on key concepts
Calculate the lattice energy of CaCl2 using a Born-Haber cycle and data from Appendices F and L and Table 7.5. ...
Chemistry & Chemical Reactivity
The following reaction has a value of G = 2.1kJ/mol(0.50kcaI/mol). CH3Br + H2S CH3 SH + HBr a. Calculate Keq a...
Organic Chemistry (9th Edition)
If white light is shined through a thin blue plastic piece, the color that will appear needs to be determined. ...
Living by Chemistry
37. Balance each redox reaction occurring in acidic aqueous solution.
a. K(s) + Cr3+(aq) → Cr(s) + K+(aq)
b. Al...
Chemistry: A Molecular Approach
In qualitative analysis, Ca2+ and Ba2+ are separated from Na+, K+, and Mg2+ by adding aqueous (NH4)2CO3 to a so...
General Chemistry: Atoms First
Practice Exercise 4.6
Nitric acid, , is a strong acid that can be used to make explosives. Nitrous acid, , is a...
Chemistry: The Molecular Nature of Matter
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1.Assign an appropriate structure (B and C) to each spectrum (l and II) below. Briefly explain your answer. 2.Assign the proton signals of compound B and C in the 1H NMR spectrum respectively, based on your answer in 1.arrow_forwardPlease draw the H-NMR spectrum for 3 of these moleculesarrow_forwarda.) Annotate the 1H - NMR spectrum by labeling each signal (A-Z) and assigning them to the correct hydrogens on the structure of your unknown molecule Annotate the 13C- NMR spectrum by labeling each signal (A-Z) and then assign the A-Z labels to the correct carbons on the structure of your unknown molecule.arrow_forward
- Please show complete answer. Answer the following questions for compounds L, M, and N drawn below. a.How many signals are expected in the 1H NMR spectrum? b.Into how many peaks is each signal in the 1H NMR spectrum split?arrow_forward2) Sketch what you would expect the 'H NMR of the following compound to look like, use labels from the structure and label correspondingly on the spectra you draw, make sure to include chemical shift, integration values and splitting patterns for your spectra. HO, A Br. D. E к Larrow_forwardBelow are the ¹H NMR spectrum of triphenylmethanol, benzophenone, and bromobenzene. Identify the compound corresponding to each ¹H NMR spectrum and draw the structure next to the ¹H NMR spectrum. Assign ALL peaks in each of the three ¹H NMR spectra. Hint: Conjugated systems (benzophenone) including an electronegative atom will cause a more downfield shift of ring protons in ¹H NMR compared with non-conjugated systems (bromobenzene). 8 8 8 7 7 7 6 6 6 5 5 5 4 PPM 4 PPM 4 PPM 3 3 3 2 2 2 1 1 1 0 0 0arrow_forward
- Select the unknown with the following information from 1H-NMR, IR and MS.arrow_forward5. A compound having molecular formula C,H,OS reacts with hydrazine hydrate forming a product C with molecular formula C₁H,N₂S₂. The ¹H-NMR and ¹C-NMR spectra of the product is given below. Identify compounds A and C. Also, assign each NMR peak. Answer as soon as possible within 10 minutes with proper explanation in handwritten form. Don't answer after 11:58 PM Otherwise I will downvote and report !! Thank youarrow_forwardUsing the molecule below, how many signals are present in its 13C NMR & 1H NMR?arrow_forward
- For the compounds below give the 1H NMR data (chemical shift, integration and multiplicity) I appreciate the help.arrow_forward5. How many signals would you expect each of the following molecules to have in its 'H NMR spectrum? Label the structure (a, b, etc...) to correlate protons with the signals in the ¹H NMR spectrums. In which regions of the spectrum would you expect to see each signal? How many hydrogen atoms will each signal integrate for? H or OMe HO Vanillin 4-hydroxy-3-methoxybenzaldehyde HO OH OMe Vanillyl Alcohol 4-hydroxy-3-methoxybenzyl alcoholarrow_forwardWith reference to the H-NMR spectra, please answer the following.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY