
Concept explainers
(a) In 1960 T. Katz (Columbia University) showed that cyclooctatetraene adds two electrons when treated with potassium metal and forms a stable, planar dianion,
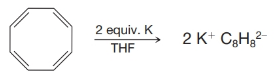
Use the molecular orbital diagram given in Fig. 14.7 and explain this result.
(b) In 1964 Katz also showed that removing two protons from the compound below (using butyllithium as the base) leads to the forma-tion of a stable dianion with the formula

Propose a reasonable structure for the product and explain why it is stable.
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Chemistry: Structure and Properties (2nd Edition)
General, Organic, and Biological Chemistry (3rd Edition)
Chemistry
Inorganic Chemistry
The Organic Chem Lab Survival Manual: A Student's Guide to Techniques
Basic Chemistry (5th Edition)
- (b) 3-methyl-2-butanol reacts with concentrated sulphuric acid to form 2-methyl-2- butene. Write the mechanism for the reaction.arrow_forward(a) Draw the structure of the following :(i) p-Methylbenzaldehyde (ii) 4-Methylpent-3-en-2-one(b) Give chemical tests to distinguish between the following pairs of compounds :(i) Benzoic acid and Ethyl benzoate, (ii) Benzaldehyde and Acetophenone.(iii) Phenol and Benzoic acid.arrow_forward(c) Arrange the following compounds in order of increasing acidity, and explain the reasons for your choice of order: phenol, cyclohexanol, 2-fluorocyclohexanol, 2-fluorophenol.arrow_forward
- Give reasons for the following: (i) p-nitrophenol is more acidic than p-methylphenol. (ii) Bond length of C—O bond in phenol is shorter than that in methanol. (iii) (CH3)3C—Br on reaction with sodium methoxide (Na+ _OCH3) gives alkene as the main product and not an ether.arrow_forwardInterpret the acidity of alcohols on the basis of ground-state polarization and stability of the alcoholate anion(indicate and give symbols for bond polarization)! Compare the relative acidity of ethanol and 2-fluoroethanol!arrow_forwardIdentify (A) in the following reaction. 2H2 Pt (A) KMNO4 Warm conc. || С — С — о—н |CO,H + HO CO2H cis-cyclo hexane 1,2-dicarboxylic acid (a) (b) (c) (d)arrow_forward
- Azulene, an isomer of naphthalene, has a remarkably large dipole moment for a hydrocarbon (µ = 1.0 D). Explain using resonance structures.arrow_forward(a) How will you carry out the following conversions?(i) Acetylene to Acetic acid (ii) Toluene to m-nitrobenzoic acid(iii) Ethanol to Acetone(b) Give reasons :(i) Chloroacetic acid is stronger than acetic acid.(ii) pH of reaction should be carefully controlled while preparing ammonia derivatives of carbonyl compounds.arrow_forwardThe enamine prepared from acetone and dimethylamine is shown here in its lowest-energy form. (a) What is the geometry and hybridization of the nitrogen atom? (b) What orbital on nitrogen holds the lone pair of electrons? (c) What is the geometric relationship between the p orbitals of the double bond and the nitrogen orbital that holds the lone pair? Why do you think this geometry represents the minimum energy?arrow_forward
- Explain why (i) the dipole moment in chlorobenzene is lower than that of cyclohexyl chloride. (ii) haloalkanes are only slightly soluble in water but dissolve easily in organic solvents.arrow_forward(a) How will you convert:(i) Benzene to acetophenone (ii) Propanone to 2-Methylpropan-2-ol(b) Give reasons :(i) Electrophilic substitution in benzoic acid takes place at meta position.(ii) Carboxylic acids are higher boiling liquids than aldehydes, ketones and alcohols of comparable molecular masses.(iii) Propanal is more reactive than propanone in nucleophilic addition reactions.arrow_forward(a) Tsomane and Nyiko were given a task of synthesising methylenecyclohexane 2. After a brief discussion with each other, Tsomane proposed Method A to synthesise 2 from cyclohexanone 1 while Nyiko proposed Method B that started from hydroxymethylcyclohexane 3. Each student believed that their proposed method is better than the other. (Scheme below) (1) Ph Ph 8*8 Ph THF A 1 Santande B H₂SO4 100 °C 3 OH Using curly arrows, provide full mechanistic details accounting how methylenecyclohexane 2 was synthesised according to both Methods A and B.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





