
Concept explainers
Interpretation:
The difference in the stabilities ofcycloheptatrienone andcyclopentadienone needs to be explained and the structure of Diels–Alder adduct of cyclopentadienoneneeds to be drawn.
Concept introduction:
In accordance with theHuckel’s rule,
Anaromatic compound contains conjugated pi bonds.
Antiaromatic compounds contain
Diels-Alder reaction is an addition reaction of a conjugated diene and an
The general reaction of Diels–Alder is as follows:
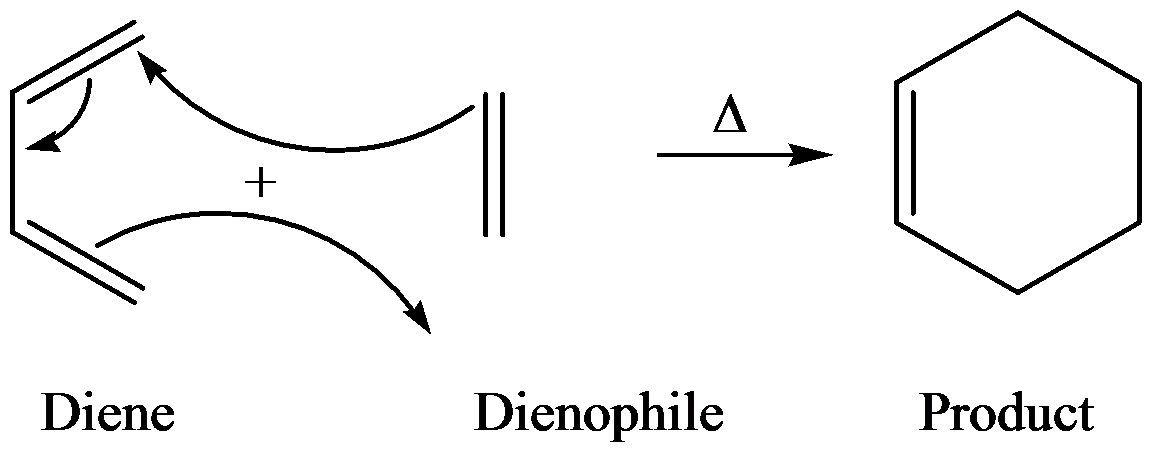
Diels Alder reaction is between a conjugate diene and a reactant containing double bond (dienophile) to form the product and the product is called adduct.
Diels Alder reactions are highly stereospecific. The configuration, if dienophile is retained in the product and the reaction, is syn addition.
The dienes react with dienophiles in cis forms rather than trans forms.
Endo and exo refer to the orientation of dienophile and its electron withdrawing group, when it reacts with a diene in Diels–Alder reaction.
The reaction with orientation of electron-withdrawing group of dienophiles under the
The reaction with orientation of electron-withdrawing group of dienophiles away from the
Endo is favored in the transition state of Diels–Alder reaction because of its lower energy.
If a compound is stable, it has lower energy and if a compound is unstable, it has higher energy.
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Organic Chemistry
- The Diels–Alder Reaction – A [4+2] Cycloaddition Experiment. (a) Explain why the endo-rule is not obeyed when furan is used as the diene. Use chemical structures and models to support your answer.arrow_forward(c) Diels-Alder reactions are a highly effective way to synthesise stereospecifically fused cyclic structures. (1) Using suitable diagrams, curly arrows and/or reaction schemes, explain why the reaction between cyclopentadiene and maleic anhydride favours formation of the endo product. (ii) If the reaction in part (i) were conducted using furan instead of cyclopentadiene, what difference would you observe in the product/s, with respect to their stereochemistry? (No need to draw reaction mechanisms) (iii) Draw the stereospecific 3D structure of the product formed during the Diels-Alder reaction below. (No need to show the reaction mechanism) Нeat Br Br (Figure Q11ciii)arrow_forward(a) The Friedel-Crafts reaction of benzene with 2-chloro-3-methylbutane in the presence of AlCl3 occurs with a carbocation rearrangement. Give mechanistic explanation and the product formed. (b) Predict the product(s) will be formed from the following reactions: (i) Bromination of p-methylbenzoic acid (ii) Sulphonation of m-bromoanisole (iii) Friedel-craft acylation of o-bromonitrobenzenearrow_forward
- One step in the synthesis of dodecahedrane (Section 4.11) involves reaction of the tetraene C with dimethylacetylene dicarboxylate (D) to afford two compounds having molecular formula C16H16O4. This reaction has been called a domino Diels–Alder reaction. Identify the two products formed.arrow_forwardOf the three 1,4-diphenyl-1,3-butadiene isomers (E,E or E,Z or Z,Z) indicate the most suitable diene that can be used as a reactant in a Diels-Alder reaction. Explain your choice.arrow_forwardThe diene lactone shown in part (a) has one electron-donating group (¬OR) and one electron-withdrawing group(C“O). This diene lactone is sufficiently electron-rich to serve as the diene in a Diels–Alder reaction.(a) What product would you expect to form when this diene reacts with methyl acetylenecarboxylate, a strong dienophile?arrow_forward
- two positions of anthracene sometimes react more like polyenes thanlike aromatic compounds.(a) Draw a Kekulé structure that shows how the reactive positions of anthracene are the ends ofa diene, appropriate for a Diels–Alder reaction.(b) The Diels–Alder reaction of anthracene with maleic anhydride is a common organic labexperiment. Predict the product of this Diels–Alder reaction.arrow_forwardWhat are the expected kinetic and thermodynamic products from addition of one mole of Br, to the following dienes? (b)arrow_forwardAccount for the following :(a) The dipole moment of chlorobenzene is lower than that of cyclohexyl chloride.(b) Alkyl halides, though polar, are immiscible with water.(c) Grignard’s reagents should be prepared under anhydrous conditions.arrow_forward
- Explain why :(a) The dipole moment of chlorobenzene is lower than that of cyclohexyl chloride.(b) Alkyl halides, though polar, are immiscible with water.arrow_forwardA chemist is attempting to synthesize a complex natural product with a highly strained cyclohexene ring system. Which type of reactants would be most suitable for achieving this goal, and why? Provide a detailed explanation of the choice of reactants and the expected outcome in terms of the Diels-Alder reaction.arrow_forwardThe following triene undergoes an intramolecular Diels-Alder reaction to give a bicyclic product. Propose a structural formula for the product. Account for the observation that the Diels-Alder reaction given in this problem takes place under milder conditions (at lower temperature) than the analogous Diels-Alder reaction 0"C Diels-Alder adductarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

