
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 27P
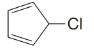
5-Chloro-1,3-cyclopentadiene (below) undergoes
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
C(CH3)2OH
C(CH3):X +H20
where (X=F, CI, Br, I) give reaction with each of halogen as well determine with which halogen
substitution reaction is more favorable and feasible and give reason of its feasibility?
Alkenyl halides such as vinyl bromide, CH2=CHBr, undergo neither SN1 nor SN2 reactions. What factors account for this lack of reactivity?
Diphenylacetylene can be synthesized by the double dehydrohalogenation of 1,2-dibromo-1,2-diphenylethene. The sequence starting from (E)-1,2-diphenylethene consists of bromination to give the dibromide, followed by dehydrohalogenation to give a vinylic bromide, then a second dehydrohalogenation to give diphenylacetylene.(a) What is the structure, including stereochemistry, of the vinylic bromide?(b) If the sequence starts with (Z)-1,2-dibromo-1,2-diphenylethene, what is (are) the structure(s) of the intermediate dibromide(s)? What is the structure of the vinylic bromide?
Chapter 14 Solutions
Organic Chemistry
Ch. 14 - PRACTICE PROBLEM 14.1 Provide a name for each of...Ch. 14 - Prob. 2PPCh. 14 - Prob. 3PPCh. 14 - Practice Problem 14.4 Apply the polygon-and-circle...Ch. 14 - Practice Problem 14.5 Apply the polygon-and-circle...Ch. 14 - Practice Problem 14.6 1,3,5-Cycloheptatriene is...Ch. 14 - Prob. 7PPCh. 14 - Prob. 8PPCh. 14 - Practice Problem 14.9 In 1967 R. Breslow (of...Ch. 14 - Prob. 10PP
Ch. 14 - Practice Problem 14.11 In addition to a signal...Ch. 14 - PRACTICE PROBLEM 14.12
Azulene has an appreciable...Ch. 14 - Practice Problem 14.13 (a) The -Sh group is...Ch. 14 - Practice Problem 14.14
Explain how NMR...Ch. 14 - PRACTICE PROBLEM 14.15 Four benzenoid compounds,...Ch. 14 - Prob. 16PCh. 14 - Write structural formulas and give acceptable...Ch. 14 - Prob. 18PCh. 14 - Prob. 19PCh. 14 - Prob. 20PCh. 14 - Which of the hydrogen atoms shown below is more...Ch. 14 - 14.22 The rings below are joined by a double bond...Ch. 14 - Prob. 23PCh. 14 - 14.24 (a) In 1960 T. Katz (Columbia University)...Ch. 14 - Prob. 25PCh. 14 - Prob. 26PCh. 14 - 14.27 5-Chloro-1,3-cyclopentadiene (below)...Ch. 14 - Prob. 28PCh. 14 - Furan possesses less aromatic character than...Ch. 14 - 14.30 For each of the pairs below, predict...Ch. 14 - Assign structures to each of the compounds A, B,...Ch. 14 - Prob. 32PCh. 14 - Give a structure for compound F that is consistent...Ch. 14 - Prob. 34PCh. 14 - Prob. 35PCh. 14 - The IR and 1H NMR spectra for compound X(C8H10)...Ch. 14 - Prob. 37PCh. 14 - Prob. 38PCh. 14 - 14.39 Given the following information, predict the...Ch. 14 - Consider these reactions: The intermediate A is a...Ch. 14 - Prob. 41PCh. 14 - Compound E has the spectral features given below....Ch. 14 - Draw all of the molecular orbitals for...Ch. 14 - Prob. 1LGPCh. 14 - Prob. 2LGPCh. 14 - 3. The NMR signals for the aromatic hydrogens of...Ch. 14 - Prob. 4LGPCh. 14 - Prob. 5LGP
Additional Science Textbook Solutions
Find more solutions based on key concepts
ConceptualPRACTICE 2.1An element is a shiny, silver-colored solid at room temperature and pressure. It conducts...
Chemistry (7th Edition)
Whether the numbering of following structure is correct or not needs to be determined and if the numbering is w...
Chemistry: Matter and Change
Q6. Express the quantity 33.2 × 104 m in mm.
a) 33.2 mm
b) 3.32 mm
c) 0.332 mm
d) 3.32 × 106 mm
Chemistry: A Molecular Approach
Fill in the blanks in the following chemical equations:
Essential Organic Chemistry (3rd Edition)
A source of electromagnetic radiation produces infrared light. Which of the following could be the wavelength ...
Chemistry: The Central Science (14th Edition)
Repeat the exercise in Problem 2.7 for the 4s and 5dx2y2 orbitals.
Inorganic Chemistry
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 15) Ignoring stereochemistry, the 1:1 reaction of chlorine with 1,3-cyclohexadiene at 25°C in the dark and in the absence of peroxide forms which of these? C1 C1 C1 6 & 60 C1 I II III IV C1 C1arrow_forward1. When cyclopentene is allowed to react with bromine in an aqueous solution of sodium chloride, the products of the reaction are trans-1,2-dibromocyclopentane, trans-1-bromo-2-chlorocyclopentane, and trans-2-bromo-1-cyclopentanol. Write mechanisms to account for the formation of all these products.arrow_forwardDetermine all of the products obtained from the addition of HCl to the 1,3-diene. Once determined, draw a mechanism that accounts for the formation of every product. Then, Identify and account for the formation of the major adduct/or adducts under these conditions assuming that the reaction is conducted under thermodynamic conditions.arrow_forward
- Compound (2E,4Z,6Z,8E)-2,4,6,8-decatetraene has been cyclized to give 7,8-dimethyl- 1,3,5- cyclooctatriene. Predict the manner of ring-closure-conrotatory or disrotatory-for both thermal and photochemical reactions and predict the stereochemistry of the product in each case. Show the outermost molecular orbital cyclizaion.arrow_forwardThe reaction of (2S)-2-chloro-3-methylpentane with sodium iodide yields two products: (2R)-2-iodo-3-methylpentane and racemic 3-iodo-2-methylpentane. Account for the formation of each of these two products. For each product, you should show how it is formed and what that tells you about the mechanism of that specific reaction.arrow_forwardFollowing is an example of a type of reaction known as a Diels-Alder reaction 1,3-Pentadiene Ethylene 3-Methylcyclohexene (a racemic mixture) The Diels-Alder reaction between a diene and an alkene is quite remarkable in that it is one of the few ways that chemists have to form two new carbon-carbon bonds in a single reaction. Given what you know about the relative strengths of carbon-carbon sigma and pi bonds, would you predict the Diels-Alder reaction to be exothermic or endothermic? Explain your reasoning.arrow_forward
- Elimination of HBr from 2-bromobutane affords a mixture of 1-butene and 2-butene. With sodium ethoxide as base, 2-butene constitutes 81% of the alkene products, but with potassium tert-butoxide, 2-butene constitutes only 67% of the alkene products. Offer an explanation for this difference.arrow_forwardWhen anthracene is added to the reaction of chlorobenzene with concentrated NaOH at 350 °C, an interesting Diels–Alderadduct of formula C20H14 results. The proton NMR spectrum of the product shows a singlet of area 2 around d 3 and abroad singlet of area 12 around d 7. Propose a structure for the product, and explain why one of the aromatic rings ofanthracene reacted as a dienearrow_forwardThe nitroso group, −N=O, is one of the few nonhalogens that is an ortho- and para-directing deactivator. Explain this behaviour by drawing resonance structures of the carbocation intermediates in ortho, meta, and para electrophilic reaction onnitrosobenzene, C6H5−N=O.arrow_forward
- Consider an E2 reaction between (2-bromoethyl)cyclohexane and base potassium tert-butoxide. Describe the rate of elimination when each of the following changes occurred: (i) The base is changed to sodium hydroxide. (ii) The halogen atom of alkyl halide is replaced by chlorine.arrow_forwardWhat aromatic product would you expect to obtain from oxidation of 1-chloro-2-ethylbenzene with potassium permanganate solution. Write the chemical name of the product as your answer. Show the reaction scheme and mechanismarrow_forwardWhen 2-bromobutane is reacted with CH3O-, two alkene products, namely 2-butene and 1-butene are obtained. Explain why the E2 reaction produces 2-butene as the major product (80%) and 1-butene as the minor product (20%)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY