
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 21P
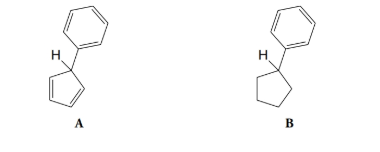
Which of the hydrogen atoms shown below is more acidic? Explain your answer.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Rank the following compounds in terms of increasing acidity (least acidic first). Explain your ranking. Making sure to say which hydrogen in each molecule is the most acidic, and discuss the relative stability of the conjugate bases.
Which one is more acidic and why?
please explain in detail with arrow pushing how you get to the answer,which hydrogen is most acidic and why?
Chapter 14 Solutions
Organic Chemistry
Ch. 14 - PRACTICE PROBLEM 14.1 Provide a name for each of...Ch. 14 - Prob. 2PPCh. 14 - Prob. 3PPCh. 14 - Practice Problem 14.4 Apply the polygon-and-circle...Ch. 14 - Practice Problem 14.5 Apply the polygon-and-circle...Ch. 14 - Practice Problem 14.6 1,3,5-Cycloheptatriene is...Ch. 14 - Prob. 7PPCh. 14 - Prob. 8PPCh. 14 - Practice Problem 14.9 In 1967 R. Breslow (of...Ch. 14 - Prob. 10PP
Ch. 14 - Practice Problem 14.11 In addition to a signal...Ch. 14 - PRACTICE PROBLEM 14.12
Azulene has an appreciable...Ch. 14 - Practice Problem 14.13 (a) The -Sh group is...Ch. 14 - Practice Problem 14.14
Explain how NMR...Ch. 14 - PRACTICE PROBLEM 14.15 Four benzenoid compounds,...Ch. 14 - Prob. 16PCh. 14 - Write structural formulas and give acceptable...Ch. 14 - Prob. 18PCh. 14 - Prob. 19PCh. 14 - Prob. 20PCh. 14 - Which of the hydrogen atoms shown below is more...Ch. 14 - 14.22 The rings below are joined by a double bond...Ch. 14 - Prob. 23PCh. 14 - 14.24 (a) In 1960 T. Katz (Columbia University)...Ch. 14 - Prob. 25PCh. 14 - Prob. 26PCh. 14 - 14.27 5-Chloro-1,3-cyclopentadiene (below)...Ch. 14 - Prob. 28PCh. 14 - Furan possesses less aromatic character than...Ch. 14 - 14.30 For each of the pairs below, predict...Ch. 14 - Assign structures to each of the compounds A, B,...Ch. 14 - Prob. 32PCh. 14 - Give a structure for compound F that is consistent...Ch. 14 - Prob. 34PCh. 14 - Prob. 35PCh. 14 - The IR and 1H NMR spectra for compound X(C8H10)...Ch. 14 - Prob. 37PCh. 14 - Prob. 38PCh. 14 - 14.39 Given the following information, predict the...Ch. 14 - Consider these reactions: The intermediate A is a...Ch. 14 - Prob. 41PCh. 14 - Compound E has the spectral features given below....Ch. 14 - Draw all of the molecular orbitals for...Ch. 14 - Prob. 1LGPCh. 14 - Prob. 2LGPCh. 14 - 3. The NMR signals for the aromatic hydrogens of...Ch. 14 - Prob. 4LGPCh. 14 - Prob. 5LGP
Additional Science Textbook Solutions
Find more solutions based on key concepts
Determine [OH], [H+], and the pH of each of the following solutions. a. 1.0 M KCl b. 1.0 M KC2H3O2
Chemistry
10.71 Identify each of the following as an acid or a base: (10.1)
H2SO4
RbOH
Ca(OH)2
HI
...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Five kilograms of a 30 wt% acetone70% water mixture is added to 3.5 kg of a 20 wt% acetone—80% MIBK mixture at ...
Elementary Principles of Chemical Processes, Binder Ready Version
3. Drag out your general chemistry book and solve any four stoichiometry problems dealing with a limiting reage...
The Organic Chem Lab Survival Manual: A Student's Guide to Techniques
49. The tabulated data show the concentration of AB versus time for this reaction:
Time(s) [AB] (M)
0 0.950
5...
Chemistry: Structure and Properties
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For each molecule below, draw the conjugate acid or conjugate base or both if the molecule hasboth a conjugate acid and a conjugate base (e.g., water).arrow_forwardWhich of these four compounds is most acidic and whyarrow_forwardChoose the more acidic for each of the following pairs. Explain the reason for your choice.arrow_forward
- Which is the least acidic? Why?arrow_forwardCould you please explain in detail for example in figure B (yours)why the hydrogen next to O is not more acidic since Oxygen is more electronegative than carbon . In your D why the oxygen you chose is more basic than the other.please explain each choice .arrow_forwardPredict which member of each pair will be more acidic. Explain your answers. 2-chloropropan-1-ol or 3-chloropropan-1-olarrow_forward
- Which compound is the most acidic? Which compound is the least acidic? Rationalize acidity based on molecular structure.arrow_forwardOne of the three molecules is much more acidic than the other two. Identify the molecule and explain why it is so much more acidic that the other two. H Harrow_forward3. Rank the hydrogens in bold in the following molecules in order of their acidity. Rank the most acidic first down to the least acidic. I > ty > IV > 161 I H II H H III IV Harrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY