
Organic Chemistry
7th Edition
ISBN: 9780321803221
Author: Paula Y. Bruice
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 37P
a. Propose a mechanism for the following reaction:
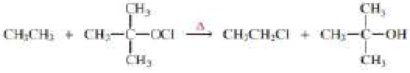
b. Given that ∆H° for the reaction is –42 kcal/mol and the bond dissociation enthalpies for the 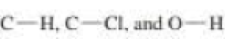 bonds are 101, 85, and 105 kcal/mol respectively, calculate the bond dissociation enthalpy of the
bonds are 101, 85, and 105 kcal/mol respectively, calculate the bond dissociation enthalpy of the 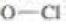 bond.
bond.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the skeletal structure of Product A from the reaction below.
COS
+
:0
heat
Product A
Given each of the following values, is the starting material or product lower in energy?
a. ΔGo = 8.0 kJ/mol
b.Keq = 10
c. ΔGo = −12 kJ/mol
d.Keq = 10−3
3.
Predict the major product(s) for the following reaction.
HCI
o°c
4. Predict the product for the following Diels-Alder reaction.
5.
Predict the product for the following Diels-Alder reaction.
ÇOCH,
A
Chapter 13 Solutions
Organic Chemistry
Ch. 13.2 - Prob. 1PCh. 13.2 - Write the steps for formation of...Ch. 13.3 - Prob. 3PCh. 13.4 - Prob. 4PCh. 13.5 - Prob. 7PCh. 13.5 - a. Would chlorination or bromination produce a...Ch. 13.5 - Prob. 10PCh. 13.6 - Prob. 11PCh. 13.7 - Prob. 12PCh. 13.7 - Prob. 13P
Ch. 13.8 - Prob. 14PCh. 13.8 - Draw the stereoisomers of the major...Ch. 13.9 - a. How many stereoisomers are formed from the...Ch. 13.9 - Prob. 17PCh. 13.9 - Prob. 19PCh. 13.9 - Prob. 20PCh. 13.9 - Prob. 21PCh. 13.10 - Prob. 22PCh. 13.11 - How many atoms share the unpaired electrons in...Ch. 13.11 - Prob. 24PCh. 13 - Prob. 25PCh. 13 - Prob. 26PCh. 13 - Prob. 27PCh. 13 - Prob. 28PCh. 13 - Prob. 29PCh. 13 - Prob. 30PCh. 13 - Prob. 31PCh. 13 - Prob. 32PCh. 13 - Prob. 33PCh. 13 - Prob. 34PCh. 13 - Prob. 35PCh. 13 - Starting with cyclohexane, how could the following...Ch. 13 - a. Propose a mechanism for the following reaction:...Ch. 13 - What stereoisomers are obtained from the following...Ch. 13 - Prob. 39PCh. 13 - Prob. 40PCh. 13 - Prob. 41PCh. 13 - Draw the products of the following reactions,...Ch. 13 - a. What five-carbon alkene forms the same product...Ch. 13 - Prob. 44PCh. 13 - Prob. 45PCh. 13 - Prob. 46PCh. 13 - Explain why the rate of bromination of methane...Ch. 13 - Prob. 48P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the reaction run at room temperature, compound I is formed. However, over prolonged reaction time, compound II becomes the major product. Which explains this phenomenon? H. но II O Compound Il is the both the thermodynamic and kinetic product. O Compound I is the kinetic product and compound II is the thermodynamic product. O Compound l is the thermodynamic product and compound Il is the kinetic product. O Compound l is the both the thermodynamic and kinetic product. O Both compounds I and II are the thermodynamic products. O Both compounds I and II are the kinetic products. %Darrow_forwardWhat is the predicted product of the reaction shown? 1. O3 2. H20 OH Он II II H. HO IV Varrow_forwardConsider the reactions below and the pool of choices. Но %3D H. O: Reagent 2 Reagent 1 Alkene 1arrow_forward
- Rank order the following in terms of relative reactivity (most reactive on the left, least reactive on the right). H. A В C Darrow_forward2. SN2 reactions to fight cancer Cyclophosphamide is a chemotherapy medication used to treat various types of cancers (i.e. lymphoma, leukemia, and breast cancer). It belongs to a group of cytotoxic alkylating agents known as Nitrogen mustards. The activity of these drugs is attributed to the chloroethylamine groups. CI R. 'N I R NH Cl + Cl R'-NH₂ Cyclophosphamide In this exercise, you will explore the mechanism responsible for the cytotoxic activity of Nitrogen mustards and how it is possible to tune their activity (therefore their toxicity) by modifying their chemical structure. R. 2.1 Identify and label all nucleophiles/electrophiles in the following reaction. `N I R chloroethylamine 2.2 Based on your above analysis, draw two possible SN2 reaction mechanisms Intermolecular reaction (reaction between reaction sites on different molecules) Intramolecular reaction (reaction between reaction sites within the same molecule) 2.3 Which of the above reaction will be faster? Why? 2.4 Copy…arrow_forwardChoose the correct answer: Predict the product(s) for the following reaction. 1. NaH, 25°C 2. CH;CH,Br II IV II IVarrow_forward
- Predict the major product of the following reaction: OH Br Br Br Br 1. H₂SO4, heat 2. HBr, -80 °Carrow_forwardCategorize each reaction below as Snl, Sn2, El or E2 88** NaSCH, HBr NaH NaOH NaSH NaOH SNZarrow_forwardWhich alkene is the main product of the following reaction? О А.Н.С О B. H с Ос. Н.С H.C H3C H.C H₂C CH3 CH₂ CH3 CH3 H3C H3C OH CH3 cat H₂SO4 Дarrow_forward
- Which of the following reactants undergoes a [3,3] sigmatropic reaction? А. он heat В. QH heat C heat D. A and Carrow_forwardChoose the correct product for each reaction. A. B. C. D. PPh3 H PPh3 || I ။arrow_forward7. The reaction of methoxide anion with bromoethane to yield the ether ethyl methyl ether and the bromide anon (Br-) is an excellent example of a general reaction type called Sy2 (substitution nucleophilic bimolecular): CH,0+ CH,СH-Br a CH3-0-CH,СH; + Br- a. Change in enthalpy is -103 kJ/mol; the change in entropy is + 0.025 kJ/mol-K. Calculate DG at 300K. b. Is the reaction endergonic or exergonic? c. Is the reaction endothermic or exothermic? d. Use curved arrows to show the complete mechanism. Reaction of 2-methyl-1-butene with H-Cl could yield TWO alkyl chloride products. Draw and name 8. them.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY