
Concept explainers
Interpretation:
The steps how the two products formed from reaction of methylenecyclohehane with NBS has to be given. Stereoisomers has to be disregarded.
Concept introduction:
Bromination of Allylic Carbons:
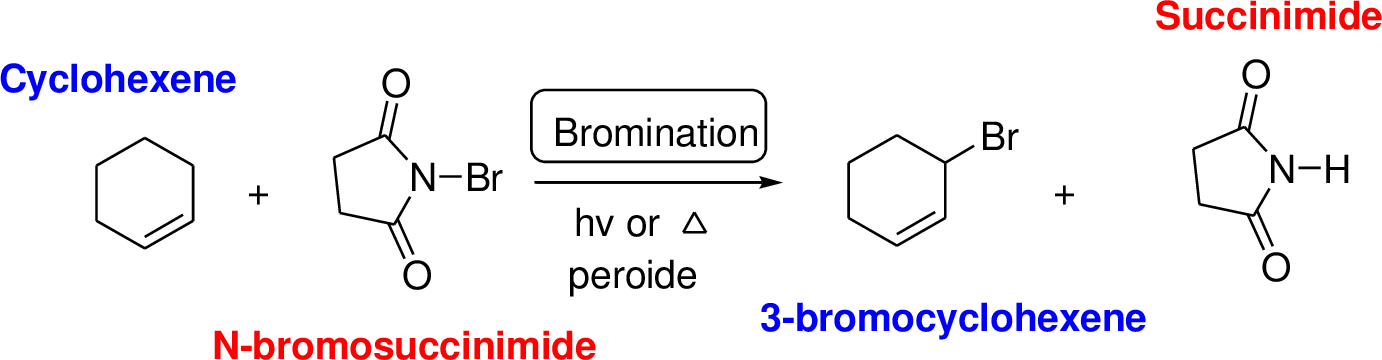
N-bromosuccinimide (NBS) is used for the allylic bromination through radical reaction. Bromination of allylicc carbon requires low concentration of bromine and low concentration of hydrobromic acid. If high concentration of bromine and high concentration of hydrobromic acid which leads to the formation of bromonation in the double bond.
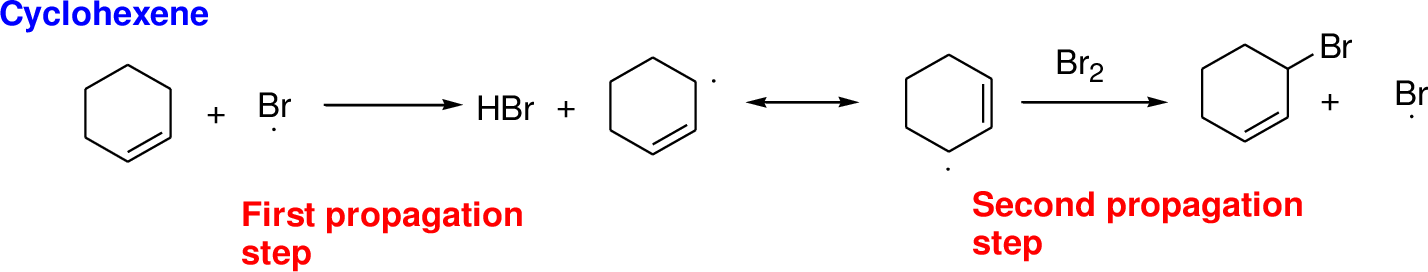
Bromination reaction starts with the homolytic cleavage of N-Br bond in N-bromosuccinimide (NBS) which creates bromine radical to initiate the radical bromination reaction.
NBS bromine radical removes the allylic hydrogen which forms hydrogen bromide and allylic radical in the first propagation step, the allylic radical is stabilized by the double bond in ring. This allylic radical reaction with bromine molecule and forms allylic bromide in the second propagation step which are shown above.
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
Organic Chemistry
- When 2-chloropropane treated with NaOH and 1-chloropropane treated with NaOH separately produce two different functional groups. Provide both reactions and explain the two different functional groups produced.arrow_forwardThe reaction of 3-methylene-1-cyclohexene and HBr yields the four products shown in the attachment. Which two are formed at high temperatures and which two are formed at low temperatures? Why? Why is 1-bromo-3-methylenecyclohexane not formed?arrow_forwardIndicate the product obtained by reacting A and B. A || H3C-S-CH₂ Na* || CH 3 B CH2. C. CH3 H3C CH3arrow_forward
- A 2-bromobutane react with methanol and form a enantiomeric pair of 2-methoxybutane. Draw the structures of the enntiomeric pairs of ethers.arrow_forwardDraw the alkene that would react with the reagent given to account for the product formed. ? + + H₂O **** H₂S04 • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. CH3 CH3 CHCCH3 | | OH CH3 +1arrow_forwardAlcohols undergo dehydration reactions in the presence of an acid catalyst. Which of the following compounds yields only a single alkene product upon dehydration?arrow_forward
- Draw the alkene that would react with the reagent given to account for the product formed. ? + HCI CH3 CH3CCH3 CI • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. #[ ] در ChemDoodlearrow_forwardGive IUPAC names for the following compounds: 1st structure: 2nd structure: SCH(CH3)2 || CH3CHCH₂CNHCH3 HCH₂CNHC Brarrow_forwardC10T05Q3420 Give the major organic product for the following reaction. H H H catalytic heat if There is no reaction under these conditions or the correct product is not listed here.arrow_forward
- Draw the organic products formed when cyclopentene is treated withfollowing reagent. [1] CH3CO3H; [2] H2O, HO−arrow_forward7. Draw a mechanism (use arrows) for the for the formation of products when 1-bromo-2-1 methylcyclohexane reacts with methanolarrow_forwardThe pictured reaction shows an alkyl bromide being converted into an alkene. Choose all reagents that would produce the pictured alkene as the major product. A) NaOH/H2O B) H2O C) tBuOK/tBuOH D) EtONa/EtOHarrow_forward
