
Concept explainers
(a)
Interpretation:
The major product obtained on treating the compounds in problem 29 with
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
Bromination:
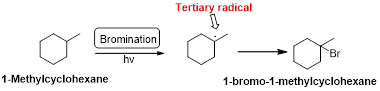
1-Methyl cyclohexane is undergoes radical bromination which forms 1-bromo-1-methyl cyclohexane therefore, bromination will occur where the tertiary radical is present. Because bromination will occur selectively in tertiary alkyl radical. (bromination reactions are more selective reaction)
(b)
Interpretation:
The major product obtained on treating the compounds in problem 29 with
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
Bromination:

1-Methylcyclohexane is undergoes radical bromination which forms 1-bromo-1-methylcyclohexane therefore, bromination will occur where the tertiary radical is present. Because bromination will occur selectively in tertiary alkyl radical. (bromination reactions are more selective reaction)
(c)
Interpretation:
The major product obtained on treating the compounds in problem 29 with
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
Bromination:
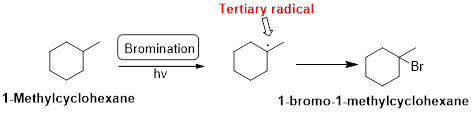
1-Methylcyclohexane is undergoes radical bromination which forms 1-bromo-1-methylcyclohexane therefore, bromination will occur where the tertiary radical is present. Because bromination will occur selectively in tertiary alkyl radical. (bromination reactions are more selective reaction)
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
Organic Chemistry
- N-Nitrosamines by themselves are not significant carcinogens. However, they are activated in the liver by a class of iron-containing enzymes (members of the cytochrome P-450 family). Activation involves the oxidation of a C-H bond next to the amine nitrogen to a C-OH group. OH ОН N=0 N=O Og 'N- H+ H N,+ cyt P-450 N-Nitroso- 2-Нydroxy-N- nitrosopiperidine An alkyl diazonium ion piperidine (a carcinogen) Show how this hydroxylation product can be transformed into an alkyl diazonium ion, an active alkylating agent and therefore a carcinogen, in the presence of an acid catalyst.arrow_forwardReaction with which of the following compounds in an acetylide reaction would lengthen the carbon chain by one carbon and add a primary alcohol? O ethanal O formaldehyde О ерохide O acetone O carbonic acid QUESTION 4 The reagent/reaction of choice for this reduction is || HC-CH=CH-CH2CH2CH2 CO2H CH2OH-CH=CH-CH2CH2CH2 CO2H O lithium aluminum hydride O potassium permanganate O sodium borohydride O Grignard reaction O SN2 reactionarrow_forwardWhat products are obtained from the reaction of the following compounds with H2CrO4 + ∆?arrow_forward
- Draw the product of the following epoxide reaction, including the stereochemistry at any stereogenic centers. H HI 25 Click and drag to start drawing a structure. X 26 :0 S ~ 27arrow_forwardWhich of the following compounds are generated by the reaction below? он (1) Hg(OAc)2, H20 но. (2) NaBHalethanol OH (A) (B) (C) (D) Compounds A, C and D O Compound B Compounds B and D O Compounds A and D (0)arrow_forwardProvide the product and mechanism for the reaction: ОН Cro, H,SO, H,0arrow_forward
- Identify the reagent(s) needed to carry out the following conversion. ОН ? OH Na metal LIAIH4 Na2Cr207, H2SO4/H2O NaOHarrow_forwardWhat is the source of the nucleophilic attack? How does the oxygen get protonated? What causes the elimination reaction?arrow_forwardWhat are the reagents of the following reaction? он ? ноarrow_forward
