
Concept explainers
(a)
Interpretation:
The electrons in energy level are to be written and electron dot structure is to be drawn.
Concept introduction:
Electrons in energy level are calculated by writing electronic configuration.
Electron dot structure is drawn by writing the symbol of the structure and putting the dots on the symbol as there are valence electrons in that element.
Answer to Problem 110A
Electronic configuration of Kr is 2, 8, 18, 8
Electron dot structure:
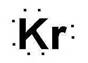
Explanation of Solution
Electronic configuration helps to draw electron dot structure. The number of dots drawn is equal to number of electrons in the valance shell.
(b)
Interpretation:
The electrons in energy level are to be written and electron dot structure is to be drawn.
Concept introduction:
Electrons in energy level are calculated by writing electronic configuration.
Electron dot structure is drawn by writing the symbol of the structure and putting the dots on the symbol as there are valence electrons in that element.
(b)
Answer to Problem 110A
Electronic configuration of Sr is 2, 8, 18, 8, 2
Electron dot structure:
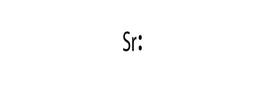
Explanation of Solution
Electronic configuration helps to draw electron dot structure. The number of dots drawn is equal to number of electrons in the valance shell.
(c)
Interpretation:
The electrons in energy level are to be written and electron dot structure is to be drawn.
Concept introduction:
Electrons in energy level are calculated by writing electronic configuration.
Electron dot structure is drawn by writing the symbol of the structure and putting the dots on the symbol as there are valence electrons in that element.
(c)
Answer to Problem 110A
Electronic configuration of P is 2, 8, 5
Electron dot structure:
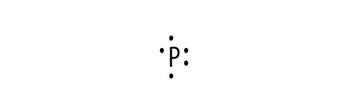
Explanation of Solution
Electronic configuration helps to draw electron dot structure. The number of dots drawn is equal to number of electrons in the valance shell.
(d)
Interpretation:
The electrons in energy level are to be written and electron dot structure is to be drawn.
Concept introduction:
Electrons in energy level are calculated by writing electronic configuration.
Electron dot structure is drawn by writing the symbol of the structure and putting the dots on the symbol as there are valence electrons in that element.
(d)
Answer to Problem 110A
Electronic configuration of B is 2, 3
Electron dot structure:
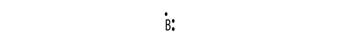
Explanation of Solution
Electronic configuration helps to draw electron dot structure. The number of dots drawn is equal to number of electrons in the valance shell.
( e)
Interpretation:
The electrons in energy level are to be written and electron dot structure is to be drawn.
Concept introduction:
Electrons in energy level are calculated by writing electronic configuration.
Electron dot structure is drawn by writing the symbol of the structure and putting the dots on the symbol as there are valence electrons in that element.
( e)
Answer to Problem 110A
Electronic configuration of Br is 2, 8, 18, 7
Electron dot structure:

Explanation of Solution
Electronic configuration helps to draw electron dot structure. The number of dots drawn is equal to number of electrons in the valance shell.
( f)
Interpretation:
The electrons in energy level are to be written and electron dot structure is to be drawn.
Concept introduction:
Electrons in energy level are calculated by writing electronic configuration.
Electron dot structure is drawn by writing the symbol of the structure and putting the dots on the symbol as there are valence electrons in that element.
( f)
Answer to Problem 110A
Electronic configuration of Se is 2, 8, 18, 6
Electron dot structure:
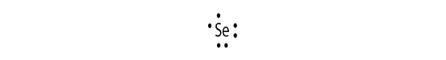
Explanation of Solution
Electronic configuration helps to draw electron dot structure. The number of dots drawn is equal to number of electrons in the valance shell.
Chapter 13 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Introductory Chemistry (5th Edition) (Standalone Book)
Chemistry: Structure and Properties (2nd Edition)
CHEMISTRY-TEXT
Organic Chemistry (9th Edition)
Essential Organic Chemistry (3rd Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





