
Concept explainers
Interpretation:
The mechanism for the given reaction has to be proposed.
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bromination of Allylic Carbons:
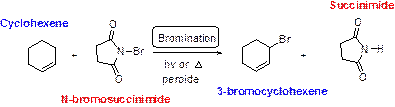
N-bromosuccinimide (NBS) is used for the allylic bromination through radical reaction. bromination of allylic carbon requires low concentration of bromine and low concentration of hydrobromic acid. If high concentration of bromine and high concentration of hydrobromic acid which leads to the formation of bromination in the double bond.
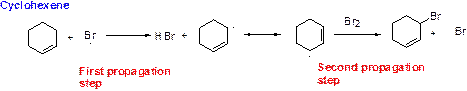
Bromination reaction starts with the homolytic cleavage of
NBS bromine radical removes the allylic hydrogen which forms hydrogen bromide and allylic radical in the first propagation step, the allylic radical is stabilized by the double bond in ring. This allylic radical reaction with bromine molecule and forms allylic bromide in the second propagation step which are shown above.
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Organic Chemistry (8th Edition)
- Pleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardThe Ksp for lead iodide ( Pbl₂) is 1.4 × 10-8. Calculate the solubility of lead iodide in each of the following. a. water Solubility = mol/L b. 0.17 M Pb(NO3)2 Solubility = c. 0.017 M NaI mol/L Solubility = mol/Larrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT


