
(a)
Interpretation: The given table needs to be completed.
Concept Introduction: From the given mass of a substance, the number of moles can be determined using molar mass as follows:
Here, m is mass and M is molar mass.
(a)
Explanation of Solution
The table shows the maximum amount of water absorbed in different quantities of
(g) | (mol) | (g) | (mol) |
| 17.3 | 5.62 | ||
| 48.8 | 15.8 | ||
| 124 | 40.3 | ||
| 337 | 109 |
The above table can be completed by calculating the number of moles of
| 17.3 | 5.62 | ||
| 48.8 | 15.8 | ||
| 124 | 40.3 | ||
| 337 | 109 |
Thus, the complete table is as follows:
| a | |||
| 17.3 | 5.62 | ||
| 48.8 | 15.8 | ||
| 124 | 40.3 | ||
| 337 | 109 |
(b)
Interpretation: The number of moles of water absorbed needs to be plotted against the number of moles of
Concept Introduction: To plot the graph of the number of moles of water absorbed versus the number of moles of
(b)
Explanation of Solution
The data is as follows:
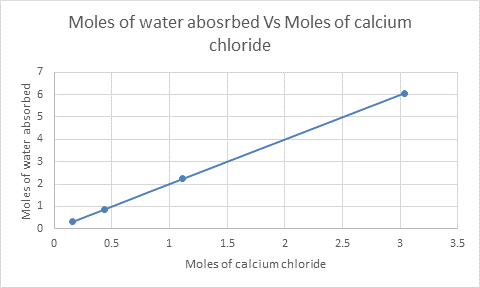
The graph can be plotted as follows:
(d)
Interpretation: Using the graph, the number of molecules of water absorbed in each formula unit of calcium chloride needs to be determined.
Concept Introduction: In 1 mol of a substance, there are
(d)
Explanation of Solution
From the graph, it is clear that 0.312 mol of water is absorbed by 0.156 mol of
The number of formula units in 0.156 mol of
Similarly, the number of water molecules in 0.312 mol of water will be:
Thus,
Therefore, 2 water molecules are absorbed by each formula unit of
Chapter 11 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





