
Concept explainers
What are intermolecular forces? How do they differ from intramolecular forces? What are dipole-dipole forces? How do typical dipole-dipole forces differ from hydrogen bonding interactions? In what ways are they similar? What are London dispersion forces? How do typical London dispersion forces differ from dipole-dipole forces? In what ways are they similar? Describe the relationship between molecular size and strength of London dispersion forces. Place the major types of intermolecular forces in order of increasing strength. Is there some overlap? That is, can the strongest London dispersion forces be greater than some dipole-dipole forces? Give an example of such an instance.
Interpretation:
- Intermolecular forces and its difference from intramolecular forces have to be described.
- Dipole-dipole forces and its variation and similarities with hydrogen bonding have to be described.
- London dispersion forces and its difference from dipole-dipole forces and similarities between them have to be explained.
- The relationship between molecular size and strength of London dispersion forces have to be described.
- Arrangement of major types of intermolecular forces in increasing order of strength has to be done.
- In case any deviation is present that has to be explained with an example.
Concept introduction:
Every atom strives to attain lowest possible energy in their shells. This is the driving force of atoms to combine with other atoms in so called “chemical reactions”. At the lowest possible energy levels, atoms and molecules attain utmost stability.
Reaching the lowest energy is not only the essential criterion for the molecules of matter to be stable. There are many other factors that have role in determining the stability of a substance. “Intermolecular forces” and “Intramolecular forces” are two such factors that have significant impact on the stability of matter.
In simple words, Intermolecular forces are termed as the forces acting “between molecules” that is components of a substance. Intramolecular forces are the forces that operate “within a molecule”. The prefix “inter” mean “among” and “intra” mean “within”.
Atoms do combine to form a molecule. Within a molecule, the atoms are held together by intramolecular forces. Many molecules are formed by such instance. Matter is composed of many such innumerable molecules which are held together by intermolecular forces. There are many types of intermolecular forces and intramolecular forces which is summarizes as follows –
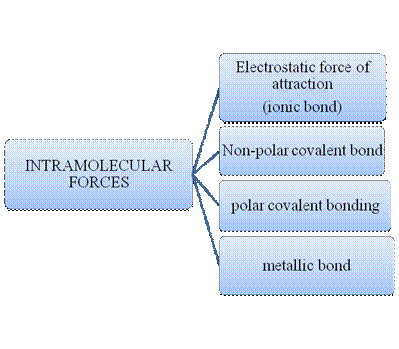
Figure 1
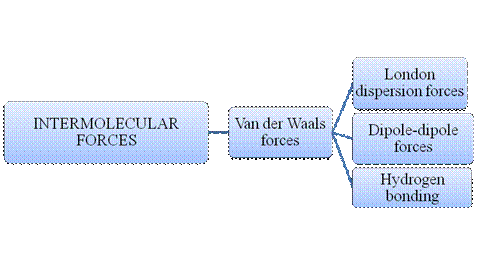
Figure 2
Intramolecular forces are nothing but the type of bonding between them. Ionic compounds have electrostatic force of attraction, the strongest one. Covalent bonds are of two types, that is polar and non-polar depend upon the polarity of the atoms. Metallic bond is formed between the metal atoms of an element.
Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
Answer to Problem 1RQ
Answer
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
- There exist no deviations in this arrangement.
Compare and contrast intermolecular forces and intramolecular forces.
Intermolecular forces are like cohesive forces, acting between the molecules whereas intramolecular forces are the forces exist within each molecule.
Explanation of Solution
Explanation
Intramolecular forces are responsible for the bond strength of a molecule. Stronger the intramolecular force stronger is the bond strength of a molecule. Stability at molecular level is highly significant.
The overall stability of a compound depends on how strong the molecules are held together. Intermolecular force is concerned about the overall stability of a substance. A stable substance has stronger intermolecular forces.
Intramolecular force is difficult to break than intermolecular force. For example, ionic bond or covalent is stronger than hydrogen bonding.
Despite, Intermolecular force is weaker than intramolecular force it has significance and impact on many of the properties of a substance.
Compare and contrast dipole-dipole forces and hydrogen bonding.
Dipole-dipole force and hydrogen bonding are the characteristic features of polar covalent compounds. Hydrogen bonding occurs only if the polar covalent compound has H-atom lies in close proximity to N, O or F whereas dipole-dipole forces exist in all polar compounds. Hydrogen bonding has high strength than dipole-dipole force.
A covalent bond is formed by mutual sharing of electrons between atoms. The two distinct types of covalent compounds are non-polar covalent and polar covalent compounds.
Atoms of the same element, particularly non-metals, bond which each other through covalent bond. There is no polarity between the atoms connected by the bond since the atoms have same electronegativity. Such type of compounds is non-polar covalent compounds. Hydrogen molecule is best example.
If atoms of slightly different electronegativity are covalently bonded, polarity arises spontaneously in the molecule due to the slight electronegativity difference between the atoms. Such compounds are polar covalent compounds. HCl molecule can be considered as example for this type.
In each molecule of a polar covalent compound, the electron cloud is displaced from the atom of low electronegativity to the atom of relatively high electronegativity through the covalent bond. As a result a “dipole” – a species containing weak partial positive and negative charge due to the unsymmetrical distribution of bonding electrons between atoms, is formed. Each dipole orient itself in such a direction that its positive end lies in close proximity to the negative end of the other dipole. The interaction between the dipoles is called “dipole-dipole forces”.
Hydrogen bonding is formed in polar covalent compounds containing hydrogen and other high electronegativity like fluorine, oxygen or nitrogen. These atoms in a molecule partially bond to hydrogen of the other same molecule or within a molecule. This type of bonding is called hydrogen bonding and it is stronger than dipole-dipole forces. Hydrogen bonding has significant impact on stability, density and other properties of matter. Water is a best example of hydrogen bonding, in which each oxygen atom of a water molecule forms hydrogen bond with hydrogen of another water molecule.
Polarity is the main reason for the formation of both the forces. Dipole-dipole force is momentarily and has no significance when the molecules are not in close proximity. But hydrogen bonding is stronger than dipole-dipole forces. Hence, hydrogen bonding is a special type of dipole-dipole forces.
Compare and contrast London dispersion forces and dipole-dipole forces.
London dispersion forces exist in non-polar compounds whereas dipole-dipole forces exist in polar covalent compounds. Dipole-dipole force is stronger than London dispersion force.
Both polar and non-polar covalent compounds have London dispersion forces. These forces are due to temporary dipoles and do not exist permanently. The molecules convert to dipoles instantly and disappear. This is due to the uneven distribution of electrons between their atoms occurs momentarily when the bonded electrons approach nucleus. Thus it is a weakest force.
On the contrary, dipole-dipole force is applicable only to polar covalent compounds. Bonding between atoms of high electronegativity difference is ionic bond. But bonding between atoms of slightly varying electronegativity is polar covalent. Thus, the molecules here exist in the form of dipole due to the uneven distribution of electrons between their atoms. The interaction between the dipoles is dipole-dipole forces and it is relative stronger than London dispersion forces. Both of them exist temporarily.
Impact of molecular size on the strength of London dispersion forces.
Molecules having large size exert London dispersion forces to the large extent.
Larger size molecules have lesser interaction between nuclei and electrons. Thus the electrons are free from nuclear force of attraction and easily form dipoles. Thus, larger the size of the molecules, higher is the strength of London dispersion force.
Arrange the main types of intermolecular forces in increasing order of its strength.
Both London dispersion forces and dipole-dipole forces do not exist permanently. London dispersion force is due to temporary dipole whereas dipole-dipole force is due to temporary dipole and remains longer time than the former one. But hydrogen bonding exists permanently and thus it is the strongest among the intermolecular forces.
Analyze whether deviation is present in the above mentioned arrangement. If deviation is found justify it with suitable example.
No deviation is present in this arrangement.
Hydrogen bonding remains as the strongest intermolecular force always. There exists no deviation in the order arrangement of their respective strengths.
Conclusion
The differences between intermolecular forces and intramolecular forces have been described. Dipole–dipole forces and its variation with hydrogen bonding have been explained. London dispersion forces and its similarities and variation with dipole-dipole forces have been explained. The relationship between size of molecules and strength of London dispersion force has been explained. The major types of intermolecular forces are arranged in increasing order of its strength. No deviation is observed in the arrangement.
Want to see more full solutions like this?
Chapter 10 Solutions
Chemistry
- Pleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardThe Ksp for lead iodide ( Pbl₂) is 1.4 × 10-8. Calculate the solubility of lead iodide in each of the following. a. water Solubility = mol/L b. 0.17 M Pb(NO3)2 Solubility = c. 0.017 M NaI mol/L Solubility = mol/Larrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





