
Concept explainers
(a)
Interpretation:
To label each bond in the compounds as ionic or covalent.
Concept introduction:
In chemistry
Ionic bond is the bond that is formed when one or more electrons from the valence shell of an atom are completely transfer to another atom valence shell. Formation of ionic bond is shown below in Fig.1.
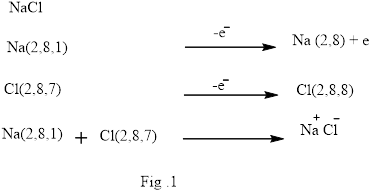
Covalent bond is formed between two atoms by mutual sharing of electrons pairs between them. By mutual sharing of electron pairs the atom attains the stable noble gas configuration. Thus in the covalent bonding the combining atom have equal claim of the shared electron pair. Illustration of covalent bond is shown below in Fig.2.
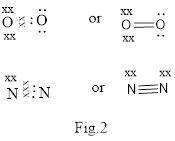
(b)
Interpretation:
To label each bond in the compounds as ionic or covalent.
Concept introduction:
In chemistry chemical bond is defined as an attractive force that contains various constituent particles (atoms, ions and molecules) in different chemical species. The chemical bond is off many types. Ionic or electrovalent bond, covalent bond and coordinate covalent bond.
Ionic bond is the bond that is formed when one or more electrons from the valence shell of an atom are completely transfer to another atom valence shell. Formation of ionic bond is shown below in Fig.1.

Covalent bond is formed between two atoms by mutual sharing of electrons pairs between them. By mutual sharing of electron pairs the atom attains the stable noble gas configuration. Thus in the covalent bonding the combining atom have equal claim of the shared electron pair. Illustration of covalent bond is shown below in Fig.2.
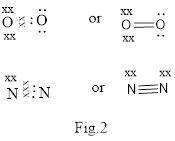
(c)
Interpretation:
To label each bond in the compounds as ionic or covalent.
Concept introduction:
In chemistry chemical bond is defined as an attractive force that contains various constituent particles (atoms, ions and molecules) in different chemical species. The chemical bond is off many types. Ionic or electrovalent bond, covalent bond and coordinate covalent bond.
Ionic bond is the bond that is formed when one or more electrons from the valence shell of an atom are completely transfer to another atom valence shell. Formation of ionic bond is shown below in Fig.1.

Covalent bond is formed between two atoms by mutual sharing of electrons pairs between them. By mutual sharing of electron pairs the atom attains the stable noble gas configuration. Thus in the covalent bonding the combining atom have equal claim of the shared electron pair. Illustration of covalent bond is shown below in Fig.2.
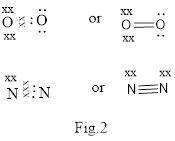
(d)
Interpretation:
To label each bond in the compounds as ionic or covalent.
Concept introduction:
In chemistry chemical bond is defined as an attractive force that contains various constituent particles (atoms, ions and molecules) in different chemical species. The chemical bond is off many types. Ionic or electrovalent bond, covalent bond and coordinate covalent bond.
Ionic bond is the bond that is formed when one or more electrons from the valence shell of an atom are completely transfer to another atom valence shell. Formation of ionic bond is shown below in Fig.1.

Covalent bond is formed between two atoms by mutual sharing of electrons pairs between them. By mutual sharing of electron pairs the atom attains the stable noble gas configuration. Thus in the covalent bonding the combining atom have equal claim of the shared electron pair. Illustration of covalent bond is shown below in Fig.2.
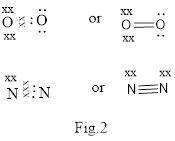
(d)
Interpretation:
To label each bond in the compounds as ionic or covalent.
Concept introduction:
In chemistry chemical bond is defined as an attractive force that contains various constituent particles (atoms, ions and molecules) in different chemical species. The chemical bond is off many types. Ionic or electrovalent bond, covalent bond and coordinate covalent bond.
Ionic bond is the bond that is formed when one or more electrons from the valence shell of an atom are completely transfer to another atom valence shell. Formation of ionic bond is shown below in Fig.1.

Covalent bond is formed between two atoms by mutual sharing of electrons pairs between them. By mutual sharing of electron pairs the atom attains the stable noble gas configuration. Thus in the covalent bonding the combining atom have equal claim of the shared electron pair. Illustration of covalent bond is shown below in Fig.2.
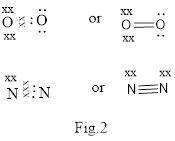
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
Organic Chemistry-Package(Custom)
- Represent the following molecules by Lewis structures: a. CH4 each H atom is bonded to the C atom b. CO2 each O atom is bonded to the C atom c. H2Se each H atom is bonded to the Se atom d. NH3 the H atom is bonded to the N atomarrow_forwardWhat main factors control the magnitude of lattice energies? Give a specific example of a compound that should have a high lattice energy, and explain why its lattice energy is high.arrow_forward1. Which is the formula of the compound formed by barium and phosphorus? a. BaP3b. Ba2P Ba3P2c. Ba3Pd. Ba2P 2. The bond between Br atoms in a Br2 molecule is _______ and is formed by the _________ of two valence electrons. a. covalent ; sharingb. covalent; transferc. ionic ; transferd. ionic ; sharingarrow_forward
- Use covalent Lewis structures to explain why each element (or family of elements) occurs as diatomic molecules.a. hydrogen b. the halogens c. oxygen d. nitrogenarrow_forwardUse the symbols &* and & to indicate the polarity of the labeled bonds. a. Br-CI b. NH,OH c. CH3, NH2 d.arrow_forward1-How many total shared electrons are there in the Lewis structure of CS2? a.10 b.6 c.2 d.8 e.4 2-In which bond does the Cl atom have the highest electron density, i.e. it attracts the electrons in the covalent bonds the most? a.N—Cl b.H—Cl c.O—Cl d.S—Cl e.Br—Clarrow_forward
- Which bond is longest?a. C-O b. C-P c. C-H d. C-C e. C-Narrow_forward1. Write the Lewis dot (electron dot) symbol for each atom or ion. a. Mg b. Mg+2 C. N d. Br¹ e. Hearrow_forwardUsing electronegativity values, classify the bond formed between each pair of elements as polar covalent or ionic. a. nitrogen and oxygen b. oxygen and hydrogen c. sulfur and chlorine d. sodium and chlorinearrow_forward
- Write the Lewis symbols for the ions in each ionic compound. c. SrBrz a. NaF b. Cao d. KOarrow_forwardLabel each bond in the following compounds as ionic or covalent. a,F2 b.LiBr c.H3CH3 d.NaNH2 e.NaOCH3arrow_forwardWhich of the following represents a non-polar covalent bond? a. H-O b. C-N c. C-C d. Li-F e. S-O Based on electronegativities, which of the following would you expect to be most ionic? a. N2 b. CaF2 c. CO2 d. CH4 e. CF4 Which element is the least electronegative? a. Calcium b. Cesium c. Iron d. Barium e. Potassiumarrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





