
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
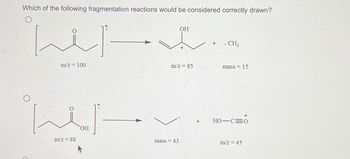
Transcribed Image Text:Which of the following fragmentation reactions would be considered correctly drawn?
ایلنا
الملينا
m/z = 100
m/z = 88
OH
m/z = 85
mass = 43
. CH3
mass = 15
HO-C=0
m/z = 45
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the following equilibrium: O || R-C-H + HCN HO OH R-C-H | CN (A) When R = CH3CH2-, Keq = 1. (i) Predict whether Keq should be greater or less than 1 when R = CICH2, and (ii) when R is CH2=CH. Explain. (B) In the case where R is CH2=CH, the cyanohydrin is formed faster but given enough time, another constitutional isomeric C4H5NO product predominates. Explain and write a base-catalyzed mechanism for the formation of the other isomer. Recall that: HO HON Narrow_forwardUse resonance/equilibria structures and explain the difference in pka. کھوچھ OH NO₂ C D OHarrow_forwardThe first step of the addition of a hydrogen halide to an alkene is the addition of H+ to an sp² carbon to form a carbocation. Predict the carbocation formed in the following reaction and draw the mechanism of its formation. Edit the reaction by drawing all steps in the appropriate boxes and connecting them with reaction arrows. Add charges where needed. Electron-flow arrows should start on an atom or a bond and should end on an atom, bond, or location where a new bond should be created. □ C MN + T [1] A H₂C -CH₂ 7 L H EXP. CONT. i ? L H-Br: Ø H с N O S CI Br I P Farrow_forward
- Please don't provide handwriting solutionarrow_forwardThe first step of the addition of a hydrogen halide to an alkene is the addition of H+ to an sp2 carbon to form a carbocation. Predict the carbocation formed in the following reaction and draw the mechanism of its formation. Edit the reaction by drawing all steps in the appropriate boxes and connecting them with reaction arrows. Add charges where needed. Electron-flow arrows should start on an atom or a bond and should end on an atom, bond, or location where a new bond should be created.arrow_forwardWhich reactions have a positive ASrxn? A(g)+B(g) → C(g) 2 A(g)+B(s) → 3 C(g) A(s) + 2 B(g) –→ C(g) O 2 A(g)+B(g) → 4 C(g)arrow_forward
- The reaction below represents what phenomenon? H,O+hv → HO•+H formation of the hydroxyl radical in the relatively unpolluted troposphere the formation of hydroxyl radical at higher altitudes destruction of the hydroxyl radical at higher altitudes the strong reactivity of the hydroxyl radicalarrow_forwardThe first step of the addition of a hydrogen halide to an alkene is the addition of H+ to an sp² carbon to form a carbocation. Predict the carbocation formed in the following reaction and draw the mechanism of its formation. Edit the reaction by drawing all steps in the appropriate boxes and connecting them with reaction arrows. Add charges where needed. Electron-flow arrows should start on an atom or a bond and should end on an atom, bond, or location where a new bond should be created. 4²² 0 0 0 1² ²0 +²²E NN H₂C -CH₂ H EXP CONT H-Br: H C N O S CI Br I Parrow_forwardFor each organic compound in the table below, enter the locant of the highlighted side chain. CH3 CH,— CH,— CH— CH, CH3 1 compound CH₂ | CH,—C− CH,—CH — CH CH3 モー CH3 CH,—CH— CH,—C— CH | CH₂ CH3 CH3 | CH3 locant of highlighted side chain 0 X Sarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY