
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
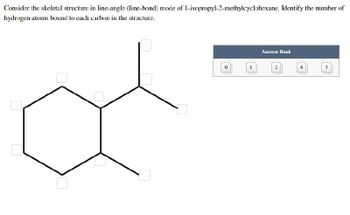
Transcribed Image Text:Consider the skeletal structure in line-angle (line-bond) mode of 1-isopropyl-2-methylcyclohexane. Identify the number of
hydrogen atoms bound to each carbon in the structure.
Answer Bank
3
I
4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Alkyl halides: find the molecular formula and the name of the molecule. Condensed Formula Molecular Formula Name CH3-CH2-CH2-CH2-I C4H9I Iodobutane CH3-CH-CH2-CH2-Br | Br ? ? CH3-CH-CH2-CH-CH2-Br | | F Br ? ?arrow_forwardOpenVellumHMAC%3D159ef0267f437acf4b/1325839e6.. 向 11of 16 Review /Constants Perlodic Table Draw the expanded structural formula for 1,3-dichlorocyclopentane An expanded struuctural formula shows all the atoms of the molecule and all the bonds between the atoms in the molecule. Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars. The single bond is active by default. Include all hydrogen atoms. View Available Hint(s) (0)arrow_forwardWrite the systematic name of each organic molecule: CH3 iO CH3 structure CH₂ CH₂-CH₂-OH OH || CH-CH-C CH3 CH3 CH3 O OH T || HỌ—CH2 CH-C CH- CH3 name 0 0arrow_forward
- Part A Indicate whether each of the following pairs represent structural isomers or the same molecule. Drag the appropriate items to their respective bins. Isomers CH, CH₂ CH CH₁ -di-di- H₂C-CH-CH-CH2 and HyC-CH-CH₂-CH-CH₂ CH₂ CH, CH₂ H₂C-C -CH₂ H–CH–CH3 and CH CH₂ + Same molecule ل۲ and Reset Helparrow_forwardWe don't see the answer written with a photo or pen, give the answer using the toolarrow_forwardName the following molecule. Group of answer choices 2-methyl-2-butane 2-methyl-2-butene 1-methyl-2-butene 3-methyl-3-butenearrow_forward
- Indicate the electron pair geometry and the molecular geometry for each of the six compounds. Compound :0=0-0: :C-S-CI: :CI-Be-Ci: CI-S-CI 0: :0=s=0: 0: H H-C-H 1 H ||||||| Electron pair geometry Molecular geometryarrow_forwardConvert this molecule into a skeletal structure and give the IUPAC name. See the image belowarrow_forwardTwo or more of your answers are incorrect. Use this condensed chemical structure to complete the table below. O || CH,=CH–C-H The condensed chemical structure of acrolein Some facts about the acrolein molecule: number of carbon-carbon single (CC) bonds: number of carbon-hydrogen single (CH) bonds: number of lone pairs: X S 2 1 4arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY