
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Bromomethylcycloheptane has been prepared in 92% yield by the reaction that it shows. Write a stepwise mechanism and use curved arrows to indicate the flow of electrons. The reaction was carried out in water, so use H3O + as the donor of protons in its mechanism. Is the determining step of the speed unimolecular (SN1) or bimolecular (SN2)?
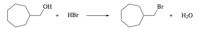
Transcribed Image Text:OH
Br
HBr
H2O
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The kinetic isotope effect for the reaction between 4-methoxyphenylethyl bromide and sodium hydroxide in aqueous solution was determined as kí/kĎ = 6.4, at room temperature. Write a reaction mechanism that is consistent with this experimental observation and explain your choice. (D)H H(D) Br OH H₂O H(D) + H₂O (HOD) + Br¯arrow_forwardThis reaction proceeds through a series of two elimination reactions. Which elimination do you believe is rate determining in this process and why?arrow_forwardConsider the reaction of the molecule below with a nucleophile (Nu). Where will the nucleophile attack in the first step of the mechanism? 0000 A B D H3C A CO1CR с B D H₂ CH3arrow_forward
- Identify the molecularity of the reaction below: 2NO(g) → N₂O₂(g) A) unimolecular B) bimolecular C) termolecular D) quadmoleculararrow_forward4. Write the free radical chain mechanism for the following bromination reaction: BrCC13 hv + Br HCC13arrow_forwardSchlapbach and Hoffmann used a stereoelectronic effect to control the stereochemistry of the reaction below. Draw the structure of the transition states for competing reactions to explain the origin of stereoselectivity. lag X = CI, OR, N(Me)SO₂R R H + Me R OH Me Xarrow_forward
- 5. In the radical addition of HBr to alkenes, we saw benzoyl peroxide as a radical initiator. The reaction below uses a different radical initiator: HBr .CN NC `N´ (radical initiator) The reaction showing the formation of the initial radicals using this initiator is shown below: CN NEN 2 х NC + NC a) Show a detailed mechanism for the reaction to produce nitrogen gas and the two carbon radicals shown above. b) Show how the carbon radical produced above could react with HBr to make a bromine radical, completing the initiation step for the reaction at the top of the page. c) Show a detailed mechanism for the propagation step of the reaction at the top of the page and circle the major organic product produced from the reaction.arrow_forwardBased on the partial reaction mechanism shown below and the reaction details, which of the following is the expected product of this reaction? H20 H. LHO H OH HOarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY