
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
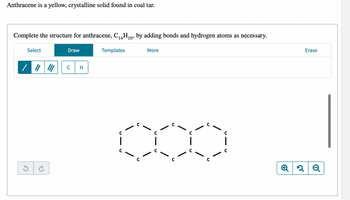
Transcribed Image Text:### Anthracene: Structure and Chemical Properties
**Background Information:**
Anthracene is a yellow, crystalline solid that is commonly found in coal tar. Its chemical formula is C₁₄H₁₀, indicating it consists of 14 carbon atoms and 10 hydrogen atoms.
**Objective:**
To complete the molecular structure of anthracene by adding the necessary bonds and hydrogen atoms.
**Instructions for Completing the Structure:**
1. **Carbon Structure Initialization:**
As depicted in the diagram below, anthracene's basic structure consists of three fused benzene rings. Each carbon atom is represented by the letter "C".
```
C C C C C C
C C C C C C
```
2. **Drawing Bonds:**
Using the drawing tools provided:
- Select the single, double, or triple bond tools as required.
- Place the bonds between the carbon atoms appropriately within the rings.
3. **Adding Hydrogen Atoms:**
- Select the "H" tool for hydrogen atoms.
- Attach hydrogen atoms to the appropriate carbons to satisfy the valence requirements (each carbon atom forms four bonds).
4. **Completion:**
Ensure all valences are satisfied with the correct number of hydrogen atoms and bonds.
**Interactive Tools:**
- **Select Tool:** Choose objects on the diagram.
- **Draw Tool:** Add bonds and atoms.
- **Templates Tool:** Use pre-defined molecular structures.
- **Erase Tool:** Remove errors or unwanted additions.
- **Undo/Redo Buttons:** Click these buttons to undo or redo your last action.
- **Zoom In/Out Buttons:** Use to get a closer or wider view of your molecular structure.
**Diagram Explanation:**
The diagram shows an incomplete structure of anthracene where only the carbon atoms forming the rings have been laid out. Each carbon atom is denoted by "C" and is connected by single or double bonds.
**Example Diagram (Benzene Rings Fused Structure):**
Below is a simplified representation of the carbon skeleton of anthracene:
```
C-C-C-C-C
/| |\
C C C
| | |
C C C
\| |/
C-C-C-C-C
```
Complete the structure by ensuring all carbons have their valences satisfied with
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Give the systematic name for each of the following organic molecules and enter it in the space provided. Be sure to include appropriate punctuation. -CI } -OH Submit Answer Tries 0/5 Br Submit Answer Tries 0/5 CI Submit Answer Tries 0/5 en Submit Answer Tries 0/5 HO Submit Answer Tries 0/5arrow_forwardDraw a Lewis structure of the following and explain how you arrived at your answerarrow_forwardTag all the tertiary sp³ carbon(s) in the structure below. If there are none, please check the box below. H H H Br No carbon (s) need to be tagged in the structure. Xarrow_forward
- Indicate the order of stability of the following species (lowest stability 1, higheststability 3)arrow_forwardPlease explain the following!arrow_forwardPart B The boiling point of another member of this homologous series was found to be 309 K. What is the likely molecular formula for this compound? Express your answer as a chemical formula. View Available Hint(s) ΑΣφ ? DA chemical reaction does not occur for this question. Submit rovide Feedback Word amazon 88 P Type here to search - 1080 acerarrow_forward
- Which of the following resonance structures for the isocyanate ion is the major resonance contributor? |:N=C—0: OOOOOOO Why twee? This C A and B are equal contributors A and C are equal contributors B and C are equal contributors A, B and C are equal contributors C=0 B ·1⁰- CEO: Oarrow_forward1-chloro-3-hexyne Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars. The single bond is active by default. H: 22 EXP" CONT 0 0 CI H2 Br CH3 [1] F P.arrow_forwardThe structure of a chiral isomer is given in the image. Draw the mirror image isomer. Use wedge-and-dash bonds for the substituent groups, and be sure that the bonds convey tetrahedral geometry. Br Но -OH Incorrect Br H illarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY