
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Assign all the protons
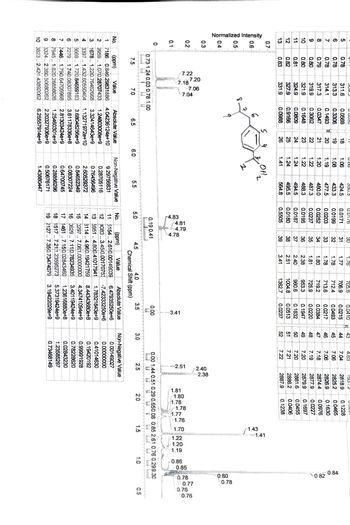
Transcribed Image Text:0
0.1
0.2
Normalized Intensity
0.3
0.4
-7.22
7.20
7.18
-7.06
-7.04
325.5
0.0050
U.00
350.5
0.0176
30
1.76
705.6
0.0410
43
4.83
1931.2
569
0.78
311.6
0.0569
18
1.06
424.5
0.0171
31
1.77
706.9
0.0215
44
7.04
2818.9
0.1228
0.78
313.3
0.3306
19
1.08
433.3
0.0199
32
1.78
712.4
0.0493
45
7.06
2825.2
0.0465
7
0.78
314.1
0.1493
20
1.19
477.5
0.0203
33
1.78
713.6
0.0217
46
7.06
2826.9
0.1630
8
0.79
317.3
0.0347
21
1.20
480.0
0.0252
34
1.80
719.2
0.0384
47
7.18
2874.4
0.0976
9
0.80
319.9
0.3062
10
0.80
321.9
0.1648
11
0.81
324.9
0.0809
12
0.82
327.9
0.9166
13
0.83
331.9
0.0988
22222
1.22
487.3
0.0237
35
1.81
725.9
0.0220
48
7.19
2877.9
0.0227
1.22
488.3
0.0195
36
2.38
953.3
0.1947
49
7.20
2879.9
0.1697
1.24
494.5
0.0187
37
2.40
960.6
0.1832
50
7.20
2881.6
0.0455
1.24
495.5
0.0180
38
2.51
1004.8
0.0513
51
7.21
2886.2
0.0406
1.41
564.8
0.5505
39
3.41
1362.7
0.0237
52
7.22
2887.9
0.1238
0.7
4
OH₂
0.6
6
3
7
0.5
8.
2
0.73 1.24 0.03 0.78
1.00
0.19 0.41
ㅂ H
7.5
7.0
6.5
6.0
5.5
5.0
4.5
4.83
4.81
-4.79
4.78
No.
(ppm)
Value
Absolute Value
Non-Negative Value
No.
1
7186.. 0.849.29831886
4.04236124e+10
9.29756831
11
Value
(ppm)
5154.. 2.610.00149039
Absolute Value
Non-Negative Value
6.47935250e+6
0.00149027
2 9959.. 1.070.28707433
1.24803008e+9
0.28705116
3
1678. 1.220.76462668
3.32414643e+9
0.76456496
12 4083.. 3.45-0.00170753
13 5951 .. 4.800.41017941
-7.42333250e+6
0.00000000
1.78321843e+9
0.41014630
4
3397.. 1.432.60550404
1.13271972e+10
2.60529372
14 8114.. 4.960.19421759
8.44343680e+8
0.19420192
5 6669.. 1.720.84659183 3.68048256e+9
0.84652349
15 0297.. 7.061.00000000
4.34741094e+9
0.99991928
6 7279. 1.740.08307895
3.61178336e+8
0.08307224
7 7446.. 1.790.64705968
2.81303424e+9
8 7945.. 1.820.28858626
9 3324.. 2.380.50680262
1.25460301e+9
2.20327936e+9
10 3823.. 2.421.43892062 6.25557914e+9
0.64700745
0.28856296
0.50676171
1.43880447
16 0629.. 7.110.78234935
17 1461..7.150.02843460
3.40119424e+9
0.78228620
1.23616880e+8
0.02843230
18 1517.. 7.211.23595273
19 2127.. 7.350.73474079
5.37319424e+9
1.23585297
3.19422029e+9
0.73468149
4.0
Chemical Shift (ppm)
3.5
0.00
ㅂ
3.0
-3.41
-2.51
-2.40
-2.38
1.77
1.76
1.70
1.43
-1.41
1.80
1.78
-1.78
1.81
1.20
1.22
1.19
-0.86
0.85
0.78
0.80
0.77
0.78
0.00 1.44 0.51 0.29 0.650.08 0.85 2.61 0.76 0.299.30
பப ㅂ ㅂ ㅂㅂ ㅂㅂㅂ
2.5
2.0
1.5
1.0
0.5
0.76
0.76
0.84
0.82
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- ALEKS - Jacqueline Hoppenrey x G convert mg to g - Google Searc x A www-awn.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IQiHqRdYV 6Ux63Syp.JXz0Coxvwqgg4JkWI72X79QvOLp9_7U27sYQhkaocvdwecGvsUzo65uy3F6spORRg1XSqgh81is O STOICHIOMETRY Using molarity to find solute mass and solution volume Jacqueline A chemist adds 55.0 mL of a 4.75M silver perchlorate (AGCIO,) solution to a reaction flask. Calculate the mass in grams of silver perchlorate the chemist has added to the flask. Round your answer to 3 significant digits. Explanation Check Privacy Accessibil 2021 McGraw-Hill Education All Rights Reserved Terms of Usearrow_forwardMolar concentration of the dye stock solution: 2.52e-5 mol/L Operating Wavelength: 630 nm Solutions(Dilutions) Absorbance (A)(a.u.) 1 0.073 2 0.118 3 0.258 4 0.477 Using linear regression determine the absorbance/concentration relationship for the dye. [dye] =_____ x Aarrow_forwardCom X Bb Mas X Mas X Hom X K! Kahx Exan X K! Kah X Mitc X КI Kah X K Kah x K! Kah x Kah x K Kahx C session.masteringchemistry.com/myct/itemView?assignmentProblemID=134214560 Apps New Tab Effect of Salinity on... Sleep Disorders | M... P Philo American Journal ... Zeeshan JBAS Ic Wait for Next Quest... oramge juice Effect carrow_forward
- Can you help me label this irarrow_forwardLearning AA prod03-cnow-owl.cengagenow.com Login Learning Learning × Online tea... y dr. marlow... ember L... with We 3. AS surroundi... M 2BrF3 (9) Br2 (g) + 3F2 (9) 4. AG° = AH°... M 5. AG: Pre... 1req 6. AG: Enthal... Using standard thermodynamic data at 298 K, calculate the free energy change when 2.14 moles of BrF3 (9) react at standard conditions. Substance AG (kJ/mol) 7. AG fro... 1req Question Question Question 8. Calculat... 1req AG° = 9. Calculat... 1req BrF3 (9) -229.4 Br2(g) 3.1 F2(9) 0.0 kjarrow_forwardAssessment-Chem 18-Gen (X 80 $ 54 R UCI General Chemistry Peer Tu X 65.2% Use the molar bond enthalpy data in the table to estimate the value of AHin for the equation CCI, (g) + 2 F₂ (g) →→→ CF₂(g) + 2Cl₂(g) The bonding in the molecules is shown. ΔΗ; = 888 F4 ++++ % 5 FS F-F A 6 New Tab MacBook Air ܀ T Y F6 & 7 CICI CI-CI A4 F7 * 8 ∞ Resources Average molar bond enthalpics. (Hond) kJ. mol Bond 464 C=N 142 N-H 351 N-N 502 N=N 730 N=N 347 F-F 615 811 414 439 331 276 293 615 Bond O-H 0-0 C-0 0=0 C=0 C-C C=C C=C C-H C-F C-CI C-Br C-N C=N DII FB 69 DD F9 kJ Question Source: McQuarrie, Rock, And Gallogly 4e-General Chemistry Publisher: University Science Books 1 0 ) CI-CI Br-Br H-H H-F H-CI H-Br H-S S-S 0 A F10 I' Check Answer P kJ. mol-1 890 390 159 418 945 155 243 192 435 565 431 368 364 225 3 F11 0 + Show All 411) F12 X }arrow_forward
- 5964. X The in xIG what x I PAP C X G 2HCI X PAP C x G Thep x Q chapt x b ar-2903012.agilixbuzz.com/student/135113422/activity/9ff34d34-df47-4faa-a022-8d87ab138ea2 G.How x Chem x G List a x HASessi X Mastery Assess It 7 150% +, Reset PAP Chemistry-2903012-42100P-1 / Stoichiometry / Lesson 107 Enrique Solis All changes saved 13. Some rocket engines use a mixture of hydrazine, N2 H4, and hydrogen peroxide, H2O2, as the propellant. The reaction equation is N2H4(0) +2 H2O2() → N2(9) + 4 H2O(). Which is the limiting reagent in this reaction when 0.750 mol N2H4 is mixed with 0.500 mol H2O2? O a. H2O b. H2O2 O c. N2H4 с. NHА O d. N2 SAVE&EXIT 13 of 16 NEXT PREVIOUS O Oarrow_forwardI 596420-7 x The impo X IG what ma X H PAP Cher X Qchapter x ぐ -> b ar-2903012.agilixbuzz.com/student/135113422/activity/9ff34d34-df47-4fas022 Mastery Assess It 7 PAP Chemistry-2903012-42100P-1/ Stoichiometry/Lesson 107 12. The combustion of glucose is represented by the following balanced equation: CGH12O6+6 0, 6 H20+6CO2. Which reactant is the limiting reagent if there is 1 gram of both 0, Hy06 and 0,7 O a. CgHy06 O b. H20 O c. 0, Od. CO,arrow_forwardCommun x Bb Blackboard Collaborate Ultra -2 X General Psychology-Fall 20 O X A ALEKS - Griffin Barden - Learn ww-awa.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-JcZzdcvSCzsqTCIDqNGV3bKqhMfPmUcQ4ENkmiXn9QCwgeDPDkQ06yszYWESPcekwL0-Qg619rekU7404HgFAGBEZaDr080?1oBw7QYjibavbSPXtx-YCjsh_7mMmrq#item O THERMOCHEMISTRY Griffin V Using Hess's Law to calculate net reaction enthalpy Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. In the first step, nitrogen and hydrogen react to form ammonia: N,(g) + 3 H,(g) → 2 NH3(g) AH=-92. kJ In the second step, ammonia and oxygen react to form nitric acid and water: NH3(g) + 20,(g) HNO3(g) H,O(g) AH=-330. kJ – + Calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. dla Round your answer to the nearest kJ. kJarrow_forward
- I need step by step process solving [4.184x156(Tf-295)] + [4.184x85.2(Tf-368)] = 0. because my work is wrong. Thank you!!arrow_forwardC 596420 X I The im X G what m X HPAP Ch x GHow m X G2HCIC X a ar-2903012.agilixbuzz.com/student/135113422/activity/9ff34d34-df47-4faa-a022-8d87ab138ca2 Chemi X Gst a x Sessio X Mastery Assess It_7 175% Reset PAP Chemistry-2903012-42100P-1/ Stoichiom... Enrique Solis All changes saved 16. How many moles of carbon dioxide are produced in the reaction between hydrochloric acid and calcium carbonate when 2 moles of HCl are used to start with? 2HCI + CaCO3 → CaCl2 + CO2 + H2O O 4 O 2 SUBMIT ALL ANS 16 of 16 SAVE & EXIT PREVIOUS 中arrow_forwardHelp 100% 47 T. "ublic Health Ch HSC 258 - Major Projec x MindTap - Cengage Lea X C The Illustration To T =55750828934189288909969212&elSBN=9781305657571&id=D1061392007&nbld=21... * Q Search t Referonces Use the References to access important values if needed for this question. For the following reaction, 50.4 grams of sulfur dioxide are allowed to react with 17.9 grams of water. sulfur dioxide (g) + water (I) sulfurous acid (H2SO3) (g) grams What is the maximum amount of sulfurous acid (H,SO3) that can be formed? What is the FORMULA for the limiting reagent? grams What amount of the excess reagent remains after the reaction is complete? Submit Answerarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY