
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Can you help me label this ir
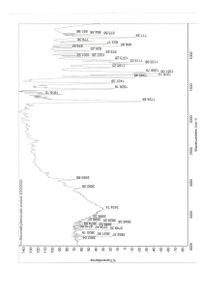
Transcribed Image Text:%Transmittance
3902.04
3852.57 3837.90 3820.19
3749.35 3734.47 3710.60
3688.53 3674.89 -
3648.38 3628.05 3586.57
33.
3424.74
3092.06
2954.88
1724.84
1616.15
1526.16
1437.39
1316.12
1287.08 1268.79
1349.48
1193.25
1133.98
1113.52
1073.28
1027.26 1001.92
975.93
925.20
858.85
879.87
823.17
779.08
717.29
675.66 664.98 651.96
Wavenumbers (cm-1)
00sz
000L
00s
000z
000s
tm-Nitromethylbenzoate product 5/20/2020
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The following statements (parts a-e) describe a specific reaction along with its regio- and/or stereo-control. Match the reaction conditions below (1-8) that correctly corresponds to the statement. Answer with 1, 2, 3, 4, 5, 6, 7, or 8. H₂ 1. Hg(OAc)2, H₂O 1. BH3, THF H₂ Lindlar's cat. Pd/C 2. NaBH4 2. H₂O₂, H₂O, OH- Condition 4 Condition 1 Condition 2 Condition 3 H₂O+ HBr NaNH, HBr H₂O2 Condition 7 Condition 8 Condition 6 Condition 5 a. Reaction condition for the reduction of an alkyne to a cis-alkene. b. Markovnikov addition of water to a double bond, with no carbocation rearrangement. c. Anti-Markovnikov addition of HBr to a double bond. d. Anti-Markovnikov addition of water to a double bond. e Reaction condition to change 1,2-dimethylcyclohexene into cis-1,2-dimethylcyclohexane.arrow_forwardUse the information in the pK table to rank the molecules in order of decreasing basicity. For exam and "2" for the next strongest base and so on. CH3NH₂ H HSO4 HS Molecules (Choose one) (Choose one) (Choose one) (Choose one) X CH1 NH₂ H₁₂ CH,NH, H₂O CH₂OH 48 38 36 33 15.7 15.5 pk₁ table a CH,NH, CH, SH 10.4 HCN NH H₂S CH,CO,H 10.6 9.4 9.2 7.00 4.76arrow_forwardHelparrow_forward
- Fill in the blank. Please choose from the following terms - copy+paste the correct term into the blank (don't alter the spelling). primary, secondary, tertiary, inversion, retention, nucleophile, base, regioselective, stereoselective, Zaitsev, Hoffman, E1, E2, antiperiplanar, trans, cis, polar aprotic, polar protic, racemic mixture, SN1, SN2 A solvent will speed up the rate of an SN2 process by many orders of magnitude.arrow_forwardfor t What is the IUPAC name of the substance shown in the following model? ball & stick v + labelsarrow_forwardPlease answer ASAParrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY