
Chemistry: Matter and Change
1st Edition
ISBN: 9780078746376
Author: Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher: Glencoe/McGraw-Hill School Pub Co
expand_more
expand_more
format_list_bulleted
Question
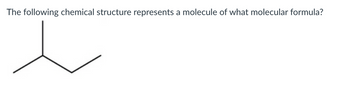
Transcribed Image Text:The following chemical structure represents a molecule of what molecular formula?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- According to the table of “Physical Constants of Inorganic Compounds", what is the formula of potassium hexachloroplatinate?arrow_forwardThe ionic compound, copper (II) hydroxide is composed ofarrow_forwardWhen atoms of and elements combine chemically, they usually electrons from their shells, and so make chemical bonds called covalent, chemical particles called and the substance composed of them calledarrow_forward
- What could the chemical formula of the molecule be?arrow_forwardHow many metal cations will be in the correct formula unit for manganese(II) phosphate?arrow_forwardChoose the correct name or formula for each of the following molecules and ionic compounds: a) magnesium nitrate ["", "", "", ""] b) iron (III) oxide ["", "", "", ""] c) FeO ["", "", "", ""] d) NO ["", "", "", ""]arrow_forward
- I do not understand what is meant by binary diatomic molecule or binary triatomic molecule or either or these formula units. What does this mean? What is an example?arrow_forwardWhat are the names of the two compounds?arrow_forwardIdentify the following two compounds as either COVALENT or IONIC: a) tetraphosphorous tetrasulfide b) potassium sulfide Cite TWO examples of evidence that the name provides which allows you to classify them in this way.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning