
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
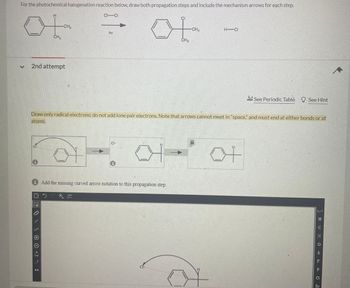
Transcribed Image Text:For the photochemical halogenation reaction below, draw both propagation steps and include the mechanism arrows for each step.
H
CH
ot
CH3
CI-CI
MM
hv
of
CH
H-CI
CH3
2nd attempt
See Periodic Table See Hint
Draw only radical electrons; do not add lone pair electrons. Note that arrows cannot meet in "space," and must end at either bonds or at
atoms.
1
i Add the missing curved arrow notation to this propagation step.
20
H
ن
S
F
P
H
CI
Br
品
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Show the mechanism for the given reaction conducted at -5 °C in CCI,. cyclohexene + bromine → dibromocyclohexane Draw structures, including charges and electrons, and add curved arrows. Details count. Draw each species (organic and inorganic) resulting from the previous step. Then, add curved arrows for the forward reaction. Include charges and nonbonding electrons. Add curved arrows to the first step. : Br - Br : Draw the major product. Include charges and nonbonding electrons.arrow_forwardPlease answer this organic chemistry question: show all three steps and radical mechanism for hydrobromic acid in peroxide reacting with 3-methylene-2-ene. Are there multiple products? Background information just in case needed for the intial question above ? but if not needed just ignore this background information: which of the following compounds will react the quickest? A) Fluorine with a tertiary radical B) chlorine with a tertiary radical C) Bromine with tertiary radical D) chlorine with a primary radical E) bromine with a primary radicalarrow_forwardProvide steps to further clarify and asure undertanding on topic.arrow_forward
- ited Draw both resonance structures of the most stable carbocation intermediate in the reaction shown. od m... • You do not have to consider stereochemistry. . Do not include anionic counter-ions, e.g., I', in your answer. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom Separate resonance structures using the → symbol from the drop-down menu. ● CH4 + ? HBrarrow_forwardThe steps shown here, which are in no particular order, have been proposed for the initiation and propagation of a radical chain-reaction mechanism. R• Cu.O + R' R' Cu• C(CH3)3 •O + + R' C(CH3)3 R' C(CH3)3 C(CH3)3 HO RH + R• + •O (a) Draw in the appropriate curved arrows for each step. (b) Label each step as either initiation or propagation. (c) Write the balanced net reaction. (d) Provide two plausible termination steps, including curved arrows. O=Uarrow_forwardIn the reaction given below, what type of reaction is used?arrow_forward
- Nitesharrow_forwardWhat is the Potential energy diagram for this reaction. Pls draw with as much detail as possible. labels and scale) 2-methylpropan-2-ol ---> 2-methylpropene +H2O Draw the potential energy diagram for the reaction of 2-methylpropan-2-ol ---> 2-methylpropene + H20 (1) Label the structures of the (a) reactants, (b) intermediates, (c) transition states, (d) products, and (e) activation energies (E.) for each step. (2) Which step is the rate-determining step? (3) Identify the electrophile and nucleophile in each step when applicable.arrow_forwardEach step is a separate reaction – you canonly use the reagents supplied for that step. For radical mechanisms, you only need to provideone termination step.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning