
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please sirrr soollveee these parts pleaseeee and thank youuuuu
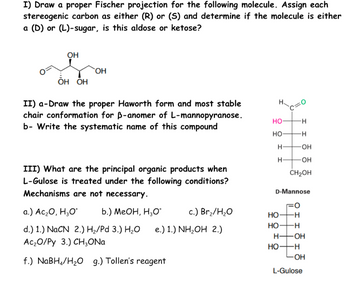
Transcribed Image Text:I) Draw a proper Fischer projection for the following molecule. Assign each
stereogenic carbon as either (R) or (S) and determine if the molecule is either
a (D) or (L)-sugar, is this aldose or ketose?
OH
OH OH
OH
II) a-Draw the proper Haworth form and most stable
chair conformation for B-anomer of L-mannopyranose.
b- Write the systematic name of this compound
III) What are the principal organic products when
L-Gulose is treated under the following conditions?
Mechanisms are not necessary.
a.) Ac₂O, H₂O* b.) MeOH, H₂O*
HO-
-H
HO-
-H
H-
-OH
H-
-OH
CH2OH
D-Mannose
c.) Br₂/H₂O
HO-
d.) 1.) NaCN 2.) H₂/Pd 3.) H₂O
e.) 1.) NH₂OH 2.)
HO-H
th
-H
Ac₂O/Py 3.) CH₂ONa
f.) NaBH4/H₂O g.) Tollen's reagent
H- -OH
но-н
LOH
L-Gulose
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 4 images

Knowledge Booster
Similar questions
- I) Draw a proper Fischer projection for the following molecule. Assign each stereogenic carbon as either (R) or (S) and determine if the molecule is either a (D) or (L)-sugar, is this aldose or ketose? ОН он он ОНarrow_forwardClassify the following monosaccharides as an aldose or ketose. I need help on number 5, a-d.arrow_forward*22) Draw the Fischer projection and Hayworth formula for D-galactose - the C4 epimer of D- glucose. (Okay to skip this for discussion with molecular models in lab.) Fischer projection of D-galactose Hayworth formula of a-D-galactose Reactions and Disaccharides 23) Aldehydes are oxidized to: 24) Open-chain aldoses can be oxidized, so they are called 'reducing sugars'. When D-glucose is oxidized, what is the carboxylic acid formed? 25) Can the hemiacetal be oxidized? 25) Can a ketose be oxidized? CHM60 Lecture Worksheet: Carbohydrates J 5 2arrow_forward
- 1 Draw D- glucose in a Fischer Projection. 2 Draw alpha-D- glucose and in the Hayworth (ring ) structure. 3 Draw the hydrolysis of maltose- the disaccharide composed of alpha D glucose molecules. 4 What is the chemical difference between cellulose and amylose NOTE- please dont explain too much explain to the pointarrow_forwardm) Which pyranose ring (A, B, C, or D) in the tetrasaccharide below is derived from D-altrose? The Fischer projections for the four aldohexoses that make up this tetrasaccharide are shown below. B C D HOH CHO CHO CHO CHO HO+H HTOH Fонно н HOH HOH H-OH H-OH H-+-OH H-TOHHOH HOH H+OH HOHнтон нон CHOH CHOH CHOH CHOH De D-glue Dalose Datrone i NH Į HOH STEP 1 OH, HO- answer 1. Just add arrows and charges for steps 1, 2, and 3 in formation of this enamine з no arrows needed here OH н Н ЮН -OH STEP 3 :ÖH н H STEP 2 на это про за почarrow_forward26. This compound is L-glyceraldehyde. Draw a stereochemically correct representation of C-1 and C-2 of D-glucose. CHO | HO—C—H | CH2OH 27. Categorize each of the following as an aldose, a ketose, or neither. 28. Define each in 20 words or less: (a) anomeric carbon; (b) enantiomers; (c) furanose and pyranose; (d) glycoside; (e) aldose and ketose. 29. A) Draw the structure of any aldohexose in the pyranose ring form. B) Draw the structure of the anomer of the aldohexose you drew above. C) How many asymmetric carbons (chiral centers) does each of these structures have? D) How many stereoisomers of the aldohexoses you drew are theoretically possible? 30. A) Define "reducing sugar." (b) Sucrose is a disaccharide composed of glucose and fructose (Glc(1 → 2)Fru). Explain why sucrose is not a reducing sugar, even though both glucose and fructose are.arrow_forward
- a) Draw Haworth projections of both - and -anomers of D-fructose. Indicate which carbon is the anomeric carbon.b) Sucrose is a disaccharide made up of a molecule of D-fructose and D-glucose. Draw the structure of sucrose clearly indicating the linkage between the two monosaccharides and its biological significance.c) Tollen’s reagent is a very mild oxidizing agent which normally oxidize aldehydes but not ketones. However, both glucose and fructose give positive results with Tollen’s reagent and are classified as reducing sugars. Explain how fructose can also give positive results with Tollen’s reagent (illustrate using structures).arrow_forward(d) Draw the structure of the expected product when monosaccharide B undergo mutarotation upon dissolving in water in the presence of Tollens reagent (AGNO3, NHẠOH). он OH O. OH OH OH monosaccharide Barrow_forwarda) Which of the following monosaccharides will react with Tollens' reagent? Circle all that apply. СООН CH2OH CH2OH OCH3 но- H- O: OH но H- но—н OH ČH2OH ČH2OH ÓH II III IV V b) Which of the following carbohydrates is sucrose? OH OH OH но OH HO, O. OH "OH HO OH Он HO OH II I HO, HO. Он HO, HO. HO, OH Но Он но. OH HO,, HO, OH HOl HO OH но он III Он HO IV HO ноarrow_forward
- Answer the following questions. (I narrowed it down to only three subparts)arrow_forward1. Draw Haworth projections of B-D-arabinofuranose and a-L-mannopyranose. 2. Consider the structure of the disaccharide drawn at right: НО `CH2 В ОН (a) Give the names and D/L designation for the two monosaccharides linked together. H,C-O OHO „OH OH А: НО НО A В: ОН (b) In the structure, circle the anomeric carbon of each saccharide. (c) Is each saccharide present in its a or ß anomer? Specify both A and B (d) Would this disaccharide undergo mutarotation? Why or why not? (e) Would this disaccharide react with Tollens and/or Benedicts reagent? Why or why not? (f) There are two reasons this is very unlikely to be a naturally occurring disaccharide. What about its structure suggests this is true? Give both reasons.arrow_forwardclassify the following monosaccharides as aldoses/ketoses, triose/tetrose/pentose/hexose/ L/D enantiomer, and a-/Banomer when applicable.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning