
Principles of Instrumental Analysis
7th Edition
ISBN: 9781305577213
Author: Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
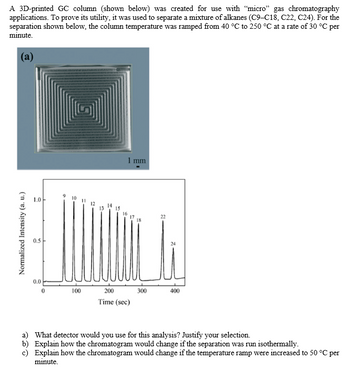
Transcribed Image Text:Normalized Intensity (a. u.)
0.5
1.0
A 3D-printed GC column (shown below) was created for use with "micro" gas chromatography
applications. To prove its utility, it was used to separate a mixture of alkanes (C9-C18, C22, C24). For the
separation shown below, the column temperature was ramped from 40 °C to 250 °C at a rate of 30 °C per
minute.
(a)
9 10
=
1 mm
12
13
15
22
0.0
0
100
200
300
400
Time (sec)
a) What detector would you use for this analysis? Justify your selection.
b) Explain how the chromatogram would change if the separation was run isothermally.
c) Explain how the chromatogram would change if the temperature ramp were increased to 50 °C per
minute.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- A3arrow_forwardA gas chromatogram of a mixture of toluene and ethyl (a) Measure w1/2 for each peak to the nearest 0.1 mm. When the thickness of the pen trace is significant relative to the length being measured, it is important to take the pen width into account. It is best to measure from the edge of one trace to the corresponding edge of the other trace, as shown at the bottom of the left column. (b) Find the number of theoretical plates and the plate height for each peak.arrow_forward(4) Below are depictions of a student's TLC plates in which fractions 1 - 10 from their column are spotted. Lane 1 2 3 4 5 67 8 9 10 (a) Which fractions contain pure fluorenone? Which contain pure ferrocene? (b) Which lanes, if any, represent mixed fractions? (c) Would the student incorporate lanes 6 and 7 into an Erlenmeyer flask of a combined fraction? Explain with 10 words or less.arrow_forward
- A young researcher set out to develop a method for quantifying the polycyclic aromatic hydrocarbon (PAH) content in a contaminated soil sample using open tubular gas chromatography. During the initial stages of method development, the researcher was unable to separate the early eluting compounds, anthracene and naphthalene, with baseline resolution. Select the possible modifications the researcher could make to improve the resolution. use a column with a smaller inner diameter decrease the column length increase the stationary phase thickness increase the temperature choose a different stationary phasearrow_forward1) Study the chromatograph (below) of a mixture of Compounds A and B, run on the GCs in the teaching labs at CU Boulder. Compound A has the shorter retention time. STAAT 61 1.11 227 RT TYPE AREA XXXX XXXX XXXX AREAS 0.009 55874 44.117 ARIHT 0.61 XX XX XX 1.11 2.27 XX XX XX TOTAL AREA=XX MUL FACTOR=XX 1. What is the retention time of compound A? Compound B? 2. Which compound is present in a larger amount? 3. Which compound has the lower boiling point? 4. What would happen to the retention times of compounds A and B if the column temperature were raised? 5. You suspect that compound B is octane. What can you do to provide supporting evidence for this hypothesis?arrow_forwardStudy the chromatograph (below) of a mixture of Compounds A and B, run on the GCs in the teaching labs at CU Boulder. Compound A has the shorter retention time. You suspect that compound B is octane. What can you do to provide supporting evidence for this hypothesis?arrow_forward
- 1. From spinach, the chromatographic analysis will yield two major substances: (a) B-carotene (C40H56), which will separate from the spinach mixture to form a yellow band, and (b) chlorophyll A (CSSH72MGN4OS), which will separate from the mixture to form a green band. i. Describe the characteristics and draw structures of these molecules. ii. From the spectrophotometric analysis, what wavelength (Amax) would you expect ß-carotene (C40H56) to absorb? What wavelength would chlorophyll A (CssH72MgN4O5) absorb?arrow_forwardPlease don't provide handwritten solution ...arrow_forward6arrow_forward
- 1carrow_forwardA gas chromatography column containing a poly(dimethylsiloxane) stationary phase is used to separate the molecules listed. Place the molecules in the order they will elute from the column. Refer to a list of retention indexes for several molecules. Elutes first hexane benzene heptane 2-pentanone 1-nitropropane octane Elutes last Anawer Bankarrow_forwardWhat is 4d?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT
