
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
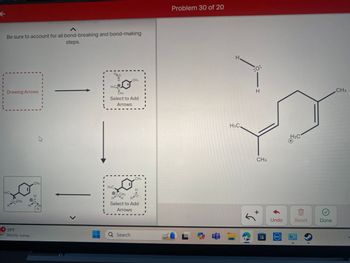
Transcribed Image Text:K
for each reaction step.
Be sure to account for all bond-breaking and bond-making
steps.
HI
HaC
Drawing Arrows!
H3C
OCH3
H
4 59°F
Mostly sunny
H
CH3
HO
O
CH3
'C'
CH3
Select to Add
Arrows
CH3 1
L
H&C.
OCH3
H H H
H
Select to Add
Arrows
Q Search
Problem 30 of 20
H.
H3C
+
:0:
H
CH3
CH3
20
H2C
Undo
Reset
Done
DELL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- NBS heat Draw the molecule(s) on the canvas by choosing buttons from the Tools (for bonds and charges), A toolbars. H± 12D EXP. CONT. H C N O S F CI Br Br P F × Incorrect; Try Again; 2 attempts remaining Note that, in the second propagation step, bromine is attacked by the least sterically hindered carbon ra Δ A. Submit Previous Answers Request Answerarrow_forwardC. Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars. The single bond is active by default. M H₂ Pd catalyst [1] 7 H 1²D EXP. CONT. i ? L 1 Marvin JS by ChemAxon H с N O S Ō Br I P Farrow_forwardShow all the bonds in the drawing please dont do ch3ch2ch draw it out tyarrow_forward
- Rewrite the entire reaction in Full Structure Format with the Ending Products [The second image is an example of Full Structure Format ] THANKSSSSSSSSSSSSarrow_forward← A → C FI 4 app.aktiv.com @ F2 (CH3CH₂)ǝN OH 80 F3 3 S PP Br U 000 000 F4 in FS S Problem 23 of 55 MacBook Air F6 g F7 A B с D E 3 C NaH DMF NaCl DMF NaHCO3 DMF NaH H₂O PP. (CH3CH₂)2N DII F8 O Done DD FO PP S PS A F10 P 4 8 AX B U F11 ★ + 0 Submit (1)) ⠀ F12arrow_forwardinvolvedinthereaction? 4.Theenthalpyforthereactionbelowis-25kJ: 2A-A+B=B→2A-B-A a.Giventhefollowingbondenergies,findthemissingvalue. b. Sketch a potential energy diagram for this reaction:arrow_forward
- Give the structure of compound A, the major product when the reaction is conducted at low temperatures. Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars. The single bond is active by default. View Available Hint(s) H 20 EXP. CONT. L C N S CI -55 °C Br Marvin JS Н — Вr [1] A by O ChemAxon F A Oarrow_forwardPredict the product for the following reaction. H₂C A I and II HH3C xoxoxox B D IV E cis-1-bromo-3-methylcyclohexane ||| SH H₂C NASH III acetone Br H3C ||||SH IV Harrow_forwardWhat type of reaction is shown below? H a. Homolytic bond cleavage ob. Elimination O. Rearrangement od. Propagation Oe. Heterolytic bond cleavagearrow_forward
- draw the major product of the following reactionarrow_forwardJators. aleacti ong 04 d 1. Predict the major Diels-Alder products of the reactions below. Indicate stereochemistry if applicable. Label the diene and dienophile. Ph. J Ph CI + * + H3CO OCH 3 CEN Pu C1 * CI осиз CH3 !!!!CENarrow_forward1. Find AHrxn for the following reaction using bond dissociation values given on the last page. Hint: You must balance the equation below. _C3H6 + _02 → _CO2 _H2O H c=Carrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY