
Interpretation : The volume of gas when it is heated to 325K is to be calculated.
Concept Introduction : According to Charles’s law, if pressure and the number of particles of a gas remain same, then volume is proportional to the Kelvin temperature.
Here, k is proportionality constant.
Answer to Problem 3E
The volume of the gas at 325K will be 687.7mL
The following graph shows the change in volume as the temperature is increased.
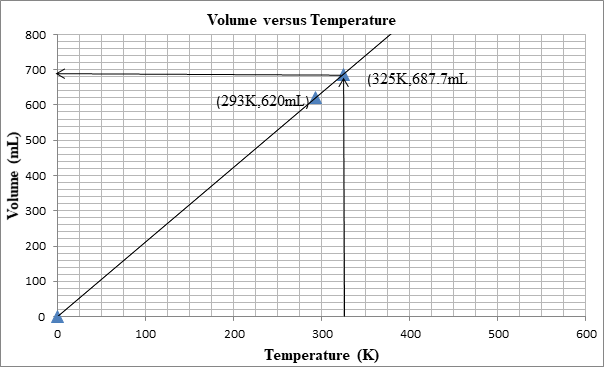
Explanation of Solution
Given information:
A sample of gas in a cylinder has a volume of 620mL at 293K. It is heated to 325K.
As the temperature increases, the volume of gas increases. Since, volume is directly proportional to temperature so one can predict that the volume will increase.
Using the formula,
One can first find the value of k,
Now solving for the volume at 325K;
The volume can be determined from the graph as well:
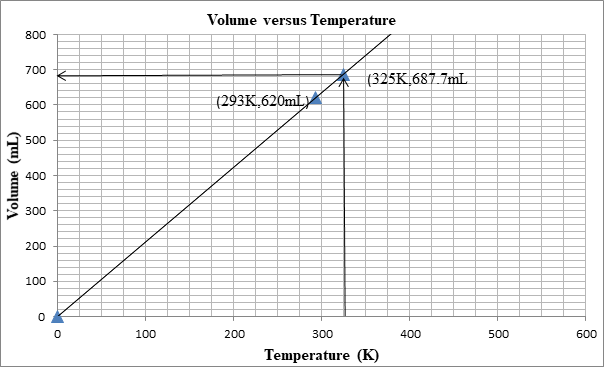
First we plot a point with initial conditions. One draws a line through this point and the origin, since at 0K the volume is theoretically 0 mL
Then one uses the line to find the volume when the x-value is 325K.
At 325K, the volume is 687mL.
Chapter U3 Solutions
Living By Chemistry: First Edition Textbook
Additional Science Textbook Solutions
Introductory Chemistry (6th Edition)
Inorganic Chemistry
Chemistry
Chemistry & Chemical Reactivity
General Chemistry: Principles and Modern Applications (11th Edition)
Chemistry: A Molecular Approach (4th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





