
Concept explainers
(a)
Interpretation: The relation between P and V as
Concept Introduction:
Due to random movement of gas particles, they colloid with other gas particles and also colloid with wall of container. The collision between gas particles of air and wall of container exert pressure on the wall of container. The gas pressure is inversely proportional to the volume of gas. This is because as the gas pressure increases, the gas particles come close to each other. It decreases the intermolecular distance between particles and volume decreases.
(a)
Answer to Problem 7E
In the given data, the constant value of k proves that:
Explanation of Solution
| Trial | Volume (mL) | Pressure (lb/in2) | Pressure (atm) | ||
| 1 | 60 mL | 10 lb/in2 | |||
| 2 | 40 mL | 15 lb/in2 | |||
| 3 | 30 mL | 20 lb/in2 | |||
| 4 | 15 mL | 40 lb/in2 | |||
| 5 | 10 mL | 60 lb/in2 |
The Boyle’s law states that at constant temperature and amount of gas molecules, the volume is inversely proportional to the pressure of gas.
In the given data, the constant value of k proves that:
(b)
Interpretation: The relation between P and V as
Concept Introduction:
Due to random movement of gas particles, they colloid with other gas particles and also colloid with wall of container. The collision between gas particles of air and wall of container exert pressure on the wall of container. The gas pressure is inversely proportional to the volume of gas. This is because as the gas pressure increases, the gas particles come close to each other. It decreases the intermolecular distance between particles and volume decreases.
(b)
Answer to Problem 7E
In the given data, the constant value of k proves that:
Explanation of Solution
The Boyle’s law states that at constant temperature and amount of gas molecules, the volume is inversely proportional to the pressure of gas.
| Trial | Volume (mL) | Pressure (lb/in2) | Pressure (atm) | |
| 1 | 60 mL | 10 lb/in2 | ||
| 2 | 40 mL | 15 lb/in2 | ||
| 3 | 30 mL | 20 lb/in2 | ||
| 4 | 15 mL | 40 lb/in2 | ||
| 5 | 10 mL | 60 lb/in2 |
(c)
Interpretation: The graph of P versus V needs to be drawn and the relation between gas pressure and volume needs to be explained.
Concept Introduction:
Due to random movement of gas particles, they colloid with other gas particles and also colloid with wall of container. The collision between gas particles of air and wall of container exert pressure on the wall of container. The gas pressure is inversely proportional to the volume of gas. This is because as the gas pressure increases, the gas particles come close to each other. It decreases the intermolecular distance between particles and volume decreases.
(c)
Answer to Problem 7E
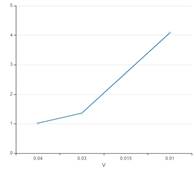
The curve between P and V interprets that with increase in pressure of gas, the volume of gas increases.
Explanation of Solution
The Boyle’s law states that at constant temperature and amount of gas molecules, the volume is inversely proportional to the pressure of gas.
| Trial | Volume (mL) | Pressure (lb/in2) | Pressure (atm) | |
| 1 | 60 mL | 10 lb/in2 | ||
| 2 | 40 mL | 15 lb/in2 | ||
| 3 | 30 mL | 20 lb/in2 | ||
| 4 | 15 mL | 40 lb/in2 | ||
| 5 | 10 mL | 60 lb/in2 |
The curve between P and V must be:
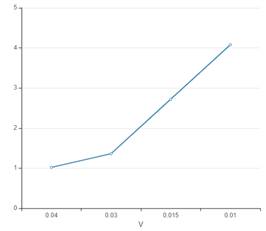
The curve between P and V interprets that with increase in pressure of gas, the volume of gas increases. Overall pressure is inversely proportional to the volume of gas.
(d)
Interpretation: The graph of P versus 1/V needs to be drawn and the relation between gas pressure and volume needs to be explained.
Concept Introduction:
Due to random movement of gas particles, they colloid with other gas particles and also colloid with wall of container. The collision between gas particles of air and wall of container exert pressure on the wall of container. The gas pressure is inversely proportional to the volume of gas. This is because as the gas pressure increases, the gas particles come close to each other. It decreases the intermolecular distance between particles and volume decreases.
(d)
Answer to Problem 7E
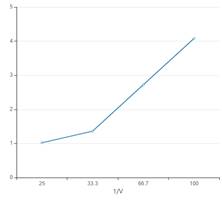
The curve between P and 1/V interprets that pressure is directly proportional to the inverse volume.
Explanation of Solution
| Trial | Volume (mL) | Pressure (lb/in2) | Pressure (atm) | |
| 1 | 60 mL | 10 lb/in2 | ||
| 2 | 40 mL | 15 lb/in2 | ||
| 3 | 30 mL | 20 lb/in2 | ||
| 4 | 15 mL | 40 lb/in2 | ||
| 5 | 10 mL | 60 lb/in2 |
The curve between P and 1/V must be:
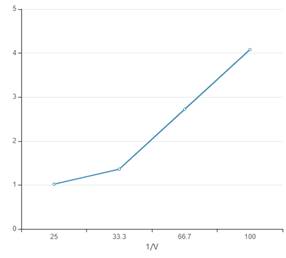
The curve between P and 1/V interprets that pressure is directly proportional to the inverse volume.
(e)
Interpretation: The volume of gas at 30 lb/in2 pressure with the help of given graph needs to be determined.
Concept Introduction:
Due to random movement of gas particles, they colloid with other gas particles and also colloid with wall of container. The collision between gas particles of air and wall of container exert pressure on the wall of container. The gas pressure is inversely proportional to the volume of gas. This is because as the gas pressure increases, the gas particles come close to each other. It decreases the intermolecular distance between particles and volume decreases.
(e)
Answer to Problem 7E
Volume at 30 lb/in2 = 0.020 L = 20.0 mL
Explanation of Solution
| Trial | Volume (mL) | Pressure (lb/in2) | Pressure (atm) |
| 1 | 60 mL | 10 lb/in2 | |
| 2 | 40 mL | 15 lb/in2 | |
| 3 | 30 mL | 20 lb/in2 | |
| 4 | 15 mL | 40 lb/in2 | |
| 5 | 10 mL | 60 lb/in2 |
Convert 30 lb/in2 to atm:
1 atm = 14.7 lb/in2
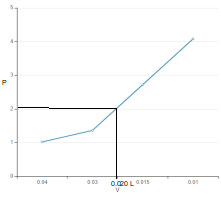
Thus the volume of gas at 2.04 atm will be 20.0 mL.
(f)
Interpretation: The pressure of 50 mL gas with the help of given graph needs to be determined.
Concept Introduction:
Due to random movement of gas particles, they colloid with other gas particles and also colloid with wall of container. The collision between gas particles of air and wall of container exert pressure on the wall of container. The gas pressure is inversely proportional to the volume of gas. This is because as the gas pressure increases, the gas particles come close to each other. It decreases the intermolecular distance between particles and volume decreases.
(f)
Answer to Problem 7E
The pressure of 0.050 L or 50.0 mL of gas must be 0.82 atm.
Explanation of Solution
| Trial | Volume (mL) | Pressure (lb/in2) | Pressure (atm) |
| 1 | 60 mL | 10 lb/in2 | |
| 2 | 40 mL | 15 lb/in2 | |
| 3 | 30 mL | 20 lb/in2 | |
| 4 | 15 mL | 40 lb/in2 | |
| 5 | 10 mL | 60 lb/in2 |
Volume = 50 mL
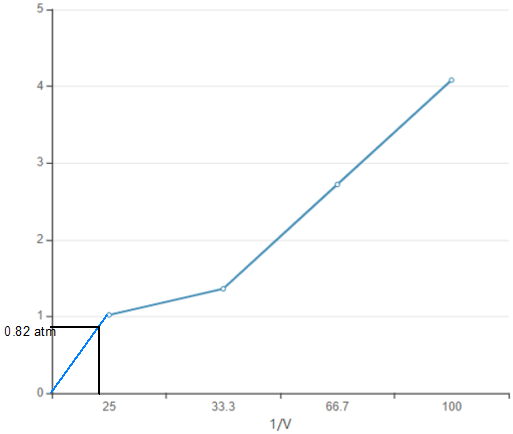
Thus the pressure of 0.050 L or 50.0 mL of gas must be 0.82 atm.
Chapter U3 Solutions
Living By Chemistry: First Edition Textbook
Additional Science Textbook Solutions
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Introductory Chemistry (5th Edition) (Standalone Book)
Essential Organic Chemistry (3rd Edition)
Chemistry (7th Edition)
Chemistry For Changing Times (14th Edition)
Chemistry: A Molecular Approach (4th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





