
Concept explainers
(a)
Interpretation:
Nuclide of an atom that has
Concept Introduction:
Complete chemical symbol notation can be given as.
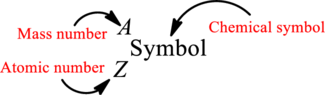
An element is a pure substance that cannot be broken by ordinary
Atomic number for each and every element is a unique one. This is the total number of protons that is present in an atom. As the atom is electrically neutral, it can also be said that the total number of electrons is the atomic number. Atomic number is represented by the symbol Z.
Mass number is the sum of the number of protons and neutrons inside the nucleus of an atom. This gives the number of subatomic particle present inside the nucleus. Mass number is represented by the symbol A.
(b)
Interpretation:
Nuclide of an atom that has
Concept Introduction:
Refer part (a).
(c)
Interpretation:
Nuclide of an atom that has
Concept Introduction:
Refer part (a).
Want to see the full answer?
Check out a sample textbook solution
Chapter F Solutions
Chemical Principles: The Quest for Insight
- Chlorine exists mainly as two isotopes, 37Cl and 33Cl. Which is more abundant? How do you know?arrow_forwardThe present average concentration (mass percent) of magnesium ions in seawater is 0.13%. A chemistry textbook estimates that if 1.00 × 108 tons Mg were taken out of the sea each year, it would take one million years for the Mg concentration to drop to 0.12%. Do sufficient calculations to either verify or refute this statement. Assume that Earth is a sphere with a diameter of 8000 mi, 67% of which is covered by oceans to a depth of 1 mi, and that no Mg is washed back into the oceans at any time.arrow_forwardGiven that the density of argon is 1.78 g/L under standard conditions of temperature and pressure, how many argon atoms are present in a room with dimensions 4.0 m 5.0 m 2.4 m that is filled with pure argon under these conditions of temperature and pressure?arrow_forward
- Assume that the radius of Earth is 6400 km, the crust is 50. km thick, the density of the crust is 3.5 g/cm3, and 25.7% of the crust is silicon by mass. Calculate the total mass of silicon in the crust of Earth.arrow_forwardBalance the following equations by filling in the blanks. (a) 92235U+01n54137_+201n+_ (b) 90232Th+612__01n+96240Cm (c) 24He+4296Mo43100_+_ (d) _+12H84210_+01narrow_forwardA sample of cocaine, C17H21O4N, is diluted with sugar, C12H22O11. When a 1.00-mg sample of this mixture is burned, 1.00 mL of carbon dioxide (d=1.80g/L) is formed. What is the percentage of cocaine in this mixture?arrow_forward
- 3.83 For the reaction of nitrogen, N2, and hydrogen, H2, to form ammonia, NH3, a student is attempting to draw a particulate diagram, as shown below. Did the student draw a correct representation of the reaction? If not, what was the error the student made?arrow_forwardWhich observations below describe chemical properties? (a) Sodium metal reacts violently with water. (b) The combustion of octane (a compound in gasoline) gives CO2 and H2O. (c) Chlorine is a green gas. (d) Heat is required to melt ice.arrow_forwardWhat is the mass of fish, in kilograms, that one would have to consume to obtain a fatal dose of mercury, if the fish contains 30 parts per million of mercury by weight? (Assume that all the mercury from the fish ends up as mercury (II) chloride in the body and that a fatal dose is 0.20 g of HgCl2.) How many pounds of fish is this?arrow_forward
- (a) Atoms are very small compared to objects on the macroscopic scale. The radius of a aluminum atom is 143 pm. What is this value in meters and in centimeters? m cm (b) The mass of a single aluminum atom is 4.48×10-23 g. Suppose enough Al atoms were lined up like beads on a string to span a distance of 37.7 cm (15 inches). How many atoms would be required? atoms What mass in grams of Al would be used? g Could you weigh out this amount of aluminum using a typical laboratory balance? (c) Taking the density of aluminum metal to be 2.70 g/cm³, calculate the mass of metal needed to form a piece of Al wire with the same length as the distance in b, but with a diameter of 1.00 mm. Hint: The volume of a cylinder is n times its radius squared times its height. (V = n r² h) How many aluminum atoms does this represent? g atomsarrow_forwardThe following diagram represents a chemical reaction in which the red spheres are oxygen atoms and the blue spheres are nitrogen atoms. (a) Write the chemical formulas for the reactants and products. (b) Write a balanced equation for the reaction. (c) Is the diagram consistent with the law of conservation of mass?arrow_forwardA compound is composed of carbon, hydrogen, nitrogen and oxygen. When a 1.500-g sample of the compound is completely combusted, it yields 1.476 g of CO2and 0.605 g of H2O. In a separate analysis to determine nitrogen, 1.500 g of the compound is found to produce 0.313 g of N2. (a) Calculate the mass percent of each element in the compound. (b) Determine the empirical formula of the compound. (c) If the compound has a molar mass of 134 g/mol, what is the molecular formula?arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





