
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
thumb_up100%
Chapter 9.5, Problem 9.6P
Predict the β-elimination product(s) formed when each chloroalkane is treated with sodium ethoxide in ethanol. If two or more products might be formed, predict which is the major product.
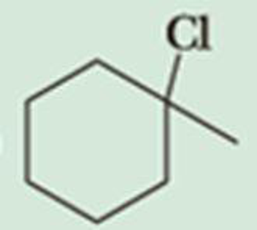
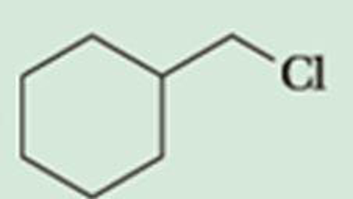
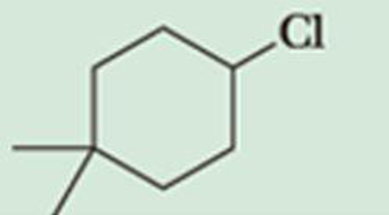
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
From this COZY spectrum, how do you know which protons are next to each other?
5. A buffer consists of 0.45 M NH, and 0.25 M NH-CI (PK of NH 474) Calculate the pH of the butter. Ans: 9.52
BAS
PH-9.26 +10g (10.95))
14-4.59
PH=4.52
6. To 500 ml of the buffer on #5 a 0.20 g of sample of NaOH was added
a Write the net ionic equation for the reaction which occurs
b. Should the pH of the solution increase or decrease sightly?
Calculate the pH of the buffer after the addition Ans: 9.54
Explain the inductive effect (+I and -I) in benzene derivatives.
Chapter 9 Solutions
Organic Chemistry
Ch. 9.1 - Prob. 9.1PCh. 9.3 - Prob. 9.2PCh. 9.3 - Prob. 9.3PCh. 9.3 - Prob. 9.4PCh. 9.4 - Prob. 9.5PCh. 9.5 - Predict the -elimination product(s) formed when...Ch. 9.7 - Prob. 9.7PCh. 9.9 - Predict whether each reaction proceeds...Ch. 9.9 - Prob. AQCh. 9.9 - Prob. BQ
Ch. 9.9 - Prob. CQCh. 9.9 - Prob. DQCh. 9.10 - Prob. 9.9PCh. 9 - Prob. 9.10PCh. 9 - Prob. 9.11PCh. 9 - Prob. 9.12PCh. 9 - Prob. 9.13PCh. 9 - Prob. 9.14PCh. 9 - Prob. 9.15PCh. 9 - Treatment of 1-aminoadamantane, C10H17N, with...Ch. 9 - Prob. 9.17PCh. 9 - Prob. 9.18PCh. 9 - Prob. 9.19PCh. 9 - Prob. 9.20PCh. 9 - Attempts to prepare optically active iodides by...Ch. 9 - Draw a structural formula for the product of each...Ch. 9 - Prob. 9.23PCh. 9 - Alkenyl halides such as vinyl bromide, CH2=CHBr,...Ch. 9 - Prob. 9.25PCh. 9 - Prob. 9.26PCh. 9 - Prob. 9.27PCh. 9 - Show how you might synthesize the following...Ch. 9 - Prob. 9.29PCh. 9 - 1-Chloro-2-butene undergoes hydrolysis in warm...Ch. 9 - Prob. 9.31PCh. 9 - Prob. 9.32PCh. 9 - Solvolysis of the following bicyclic compound in...Ch. 9 - Which compound in each set undergoes more rapid...Ch. 9 - Prob. 9.35PCh. 9 - Prob. 9.36PCh. 9 - Draw structural formulas for the alkene(s) formed...Ch. 9 - Prob. 9.38PCh. 9 - Following are diastereomers (A) and (B) of...Ch. 9 - Prob. 9.40PCh. 9 - Elimination of HBr from 2-bromonorbornane gives...Ch. 9 - Which isomer of 1-bromo-3-isopropylcyclohexane...Ch. 9 - Prob. 9.43PCh. 9 - Prob. 9.44PCh. 9 - Draw a structural formula for the major organic...Ch. 9 - When cis-4-chlorocyclohexanol is treated with...Ch. 9 - Prob. 9.47PCh. 9 - The Williamson ether synthesis involves treatment...Ch. 9 - The following ethers can, in principle, be...Ch. 9 - Prob. 9.50PCh. 9 - Prob. 9.51PCh. 9 - Prob. 9.52PCh. 9 - Prob. 9.53PCh. 9 - Prob. 9.54PCh. 9 - Write the products of the following sequences of...Ch. 9 - Using your reaction roadmap as a guide, show how...Ch. 9 - Using your reaction roadmap as a guide, show how...Ch. 9 - Using your reaction roadmap as a guide, show how...Ch. 9 - Prob. 9.59PCh. 9 - Another important pattern in organic synthesis is...Ch. 9 - Using your reaction roadmap as a guide, show how...Ch. 9 - Prob. 9.62PCh. 9 - Prob. 9.63PCh. 9 - Prob. 9.64P
Additional Science Textbook Solutions
Find more solutions based on key concepts
True or false? Some trails are considered vestigial because they existed long ago.
Biological Science (6th Edition)
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties (2nd Edition)
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
11. In the early 1800s, French naturalist Jean Baptiste Lamarck suggested that the best explanation for the rel...
Campbell Biology: Concepts & Connections (9th Edition)
Why are mutants used as test organisms in the Ames test?
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The inductive effect (+I and -I) in benzene derivatives, does it guide ortho, meta or para?arrow_forward19.57 Using one of the reactions in this chapter, give the correct starting material (A-L) needed to produce each structure (a-f). Name the type of reaction used. (b) ہ مرد (d) HO (c) དང་ ་་ཡིན་ད་དང་ (f) HO Br B D of oli H J Br K C 人 ↑arrow_forwardInductive effect (+I and -I) in benzene derivatives.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY