
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9, Problem 9.16P
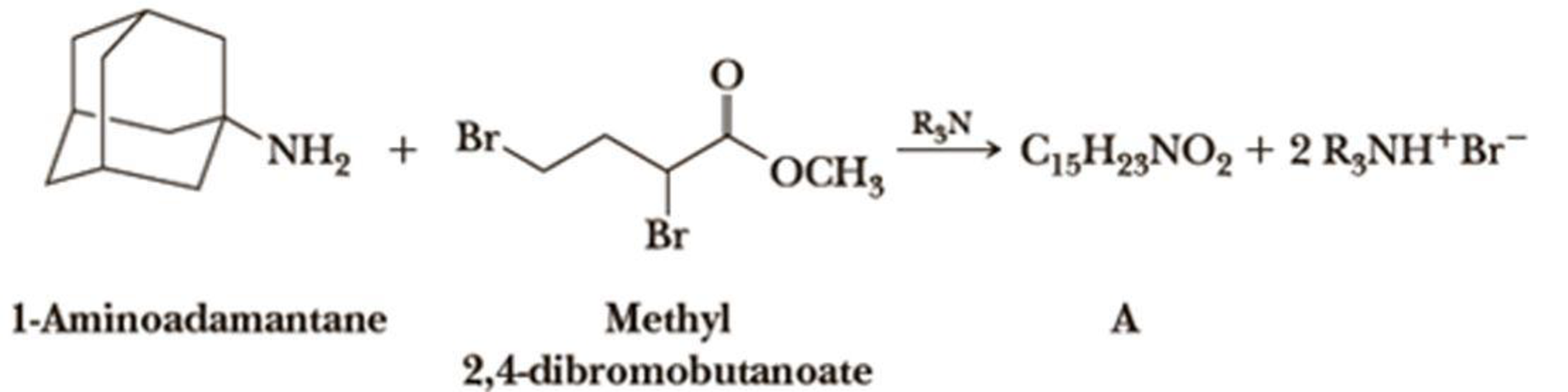
Treatment of 1-aminoadamantane, C10H17N, with methyl 2,4-dibromobutanoate in the presence of a nonnucleophilic base, R3N, involves two successive SN2 reactions and gives compound A. Propose a structural formula for compound A.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Please correct answer and don't used hand raiting
Please correct answer and don't used hand raiting
Please correct answer and don't used hand raiting
Chapter 9 Solutions
Organic Chemistry
Ch. 9.1 - Prob. 9.1PCh. 9.3 - Prob. 9.2PCh. 9.3 - Prob. 9.3PCh. 9.3 - Prob. 9.4PCh. 9.4 - Prob. 9.5PCh. 9.5 - Predict the -elimination product(s) formed when...Ch. 9.7 - Prob. 9.7PCh. 9.9 - Predict whether each reaction proceeds...Ch. 9.9 - Prob. AQCh. 9.9 - Prob. BQ
Ch. 9.9 - Prob. CQCh. 9.9 - Prob. DQCh. 9.10 - Prob. 9.9PCh. 9 - Prob. 9.10PCh. 9 - Prob. 9.11PCh. 9 - Prob. 9.12PCh. 9 - Prob. 9.13PCh. 9 - Prob. 9.14PCh. 9 - Prob. 9.15PCh. 9 - Treatment of 1-aminoadamantane, C10H17N, with...Ch. 9 - Prob. 9.17PCh. 9 - Prob. 9.18PCh. 9 - Prob. 9.19PCh. 9 - Prob. 9.20PCh. 9 - Attempts to prepare optically active iodides by...Ch. 9 - Draw a structural formula for the product of each...Ch. 9 - Prob. 9.23PCh. 9 - Alkenyl halides such as vinyl bromide, CH2=CHBr,...Ch. 9 - Prob. 9.25PCh. 9 - Prob. 9.26PCh. 9 - Prob. 9.27PCh. 9 - Show how you might synthesize the following...Ch. 9 - Prob. 9.29PCh. 9 - 1-Chloro-2-butene undergoes hydrolysis in warm...Ch. 9 - Prob. 9.31PCh. 9 - Prob. 9.32PCh. 9 - Solvolysis of the following bicyclic compound in...Ch. 9 - Which compound in each set undergoes more rapid...Ch. 9 - Prob. 9.35PCh. 9 - Prob. 9.36PCh. 9 - Draw structural formulas for the alkene(s) formed...Ch. 9 - Prob. 9.38PCh. 9 - Following are diastereomers (A) and (B) of...Ch. 9 - Prob. 9.40PCh. 9 - Elimination of HBr from 2-bromonorbornane gives...Ch. 9 - Which isomer of 1-bromo-3-isopropylcyclohexane...Ch. 9 - Prob. 9.43PCh. 9 - Prob. 9.44PCh. 9 - Draw a structural formula for the major organic...Ch. 9 - When cis-4-chlorocyclohexanol is treated with...Ch. 9 - Prob. 9.47PCh. 9 - The Williamson ether synthesis involves treatment...Ch. 9 - The following ethers can, in principle, be...Ch. 9 - Prob. 9.50PCh. 9 - Prob. 9.51PCh. 9 - Prob. 9.52PCh. 9 - Prob. 9.53PCh. 9 - Prob. 9.54PCh. 9 - Write the products of the following sequences of...Ch. 9 - Using your reaction roadmap as a guide, show how...Ch. 9 - Using your reaction roadmap as a guide, show how...Ch. 9 - Using your reaction roadmap as a guide, show how...Ch. 9 - Prob. 9.59PCh. 9 - Another important pattern in organic synthesis is...Ch. 9 - Using your reaction roadmap as a guide, show how...Ch. 9 - Prob. 9.62PCh. 9 - Prob. 9.63PCh. 9 - Prob. 9.64P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (a) The following synthesis of the molecule shown in the circle has a major problem. What is this problem? (2 pts) 1) HBr (no peroxides) 2) H- NaNH2 Br 3) NaNH, 4) CH3Br 5) H2, Pd (b) Starting with the molecule shown below and any other materials with two carbons or less, write out an alternate synthesis of the circled molecule. More than one step is needed. Indicate the reagent(s) and the major product in all the steps in your synthesis. (5 pts) 2024 Fall Term (1) Organic Chemistry 1 (Lec) CHEM 22204 02[6386] (Hunter College) (c) Using the same starting material as in part (b) and any other materials win two carpons or less, write out syntheses of the circled molecules shown below. More than one step is needed in each case. Indicate the reagent(s) and the major product in all the steps in your synthesis. You may use reactions and products from your synthesis in part (b). (5 pts)arrow_forwardalt ons for Free Response Questions FRQ 1: 0/5 To spectrophotometrically determine the mass percent of cobalt in an ore containing cobalt and some inert materials, solutions with known [Co?) are prepared and absorbance of each of the solutions is measured at the wavelength of optimum absorbance. The data are used to create a calibration plot, shown below. 0.90- 0.80- 0.70 0.60 0.50 0.40- 0.30 0.20- 0.10- 0.00- 0.005 0.010 Concentration (M) 0.015 A 0.630 g sample of the ore is completely dissolved in concentrated HNO3(aq). The mixture is diluted with water to a final volume of 50.00 ml. Assume that all the cobalt in the ore sample is converted to Co2+(aq). a. What is the [Co2] in the solution if the absorbance of a sample of the solution is 0.74? 13 ✗ b. Calculate the number of moles of Co2+(aq) in the 50.00 mL solution. 0.008 mols Coarrow_forwardPlease correct answer and don't used hand raitingarrow_forward
- Closo-boranes and arachno-boranes are structures that exhibit B-B, B-H-B, and B-H bonds. Correct?arrow_forwardIndicate why boron hydrides cannot form large linear or planar structures.arrow_forwardNido-boranes are structures with the molecular formula BnHn+4 that exhibit B-B, B-H-B and B-H bonds. Correct?arrow_forward
- 8:07 AM Wed Dec 18 Final Exam 2024 copy Home Insert Draw Page Layout Formulas Data Review AA 田 General A G fx Alexis Cozort ☑ ⚫ 61% A B D E F H K M N P R S T U 3+ 10 125 mM that yielded peak heights of Aa = 9 1-(a)A sample solution was examined under XRF to quantify the analyte Ce³+. Find the response factor F, when standardized concentration of analyte [Ce³+]A = concentration of internal standard S i.e. [In³*]s = 151 mM was spiked with standardized 1600 and As = 3015 respectively? 11 12 (i)Define F, F = Aa As [A] [S] + X 13 (*Define with variables) 4000 14 15 (ii)Calculate F, F = numeral (You will use the F value in part 1-(b) below) As 16 (*Calculate with numerals) 17 18 1-(b)To determine the unknown conc of analyte [Ce³+], a volume of 15 mL of internal standard S having a concentration [In³+]s = 0.264 M 19 20 was added to 45 mL of unknown, and the mixture was diluted to 100 mL in a volumetric flask. XRF analysis yielded a spectrum, Figure-1, where peak heights A and As are…arrow_forwardAll structural types of Boron hydrides exhibit B-B, B-H-B and B-H bonds. Correct?arrow_forwardN-nitrosodimethylamine (NDMA) is a suspected carcinogen that can form via reactions between dimethylamine (DMA) and monochloramine (NH2Cl). The relevant elementary reactions and the corresponding rate constants are as shown below. Reaction Rate constant (M¹s¹) DMA + NH2Cl = DMCA + NH3 k =1.4×10-1, kr = 5.83×10-3 1.28×10-3 DMA + NH2Cl → UDMH UDMH + NH2Cl → NDMA -> 1.11×10-1 If the initial concentrations of DMA and NH2Cl are given, you should be able to predict the concentrations of all species at any given reaction time. Please write down the rate equations for DMA, NH2C1, DMCA, UDMH and NDMA.arrow_forward
- You wish to add enough NaOCl (sodium hypochlorite) to a 150 m³ swimming pool to provide a dose of 5.0 mg/L TOTOCI as Cl2. (a) How much NaOCI (kg) should you add? (Note: the equivalent weight of NaOCl is based on the reaction: NaOCl + 2H + 2 e→CI + Na +H₂O.) (10 pts) (atomic weight: Na 23, O 16, C1 35.5) (b) The pH in the pool after the NaOCl addition is 8.67. To improve disinfection, you want at least 90% of the TOTOCI to be in the form of HOCI (pKa 7.53). Assuming that HOCI/OCI is the only weak acid/base group in solution, what volume (L) of 10 N HCl must be added to achieve the goal? (15 pts) Note that part a) is a bonus question for undergraduate students. If you decide not to work on this part of the question, you many assume TOTOCI = 7×10-5 M for part b).arrow_forwardPart A 2K(s)+Cl2(g)+2KCI(s) Express your answer in grams to three significant figures. Part B 2K(s)+Br2(1)→2KBr(s) Express your answer in grams to three significant figures. Part C 4Cr(s)+302(g)+2Cr2O3(s) Express your answer in grams to three significant figures. Part D 2Sr(s)+O2(g) 2SrO(s) Express your answer in grams to three significant figures. Thank you!arrow_forwardA solution contains 10-28 M TOTCO3 and is at pH 8.1. How much HCI (moles per liter of solution) is required to titrate the solution to pH 7.0? (H2CO3: pKa1=6.35, pKa2=10.33)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License