
(a)
Interpretation:
The member that rapidly undergoes
Concept Introduction:
The
The first step of
Frist step is the slow step also rate determining step so the rate of the reaction is depends on the concentration of substrate only.
Nucleophile attacks the both front and back side of carbocation in
Order of the substrate that favored in
(a)
Answer to Problem 9.25P
The member that rapidly undergoes
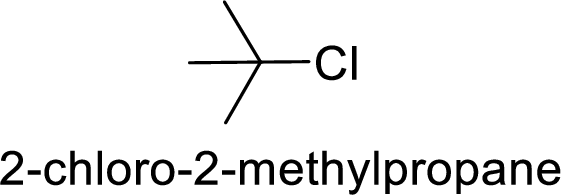
Explanation of Solution
Given:
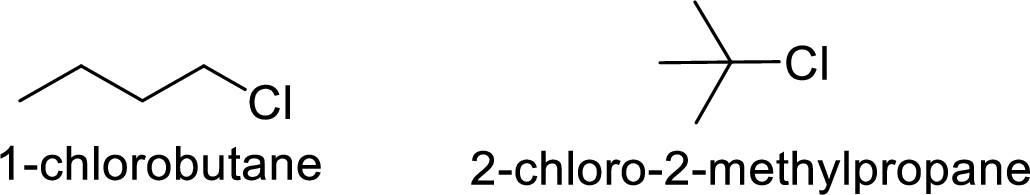
In
2-chloro-2 methyl propane gives more stable carbocation than 1-chlorobutane.
Hence, the member that rapidly undergoes
(b)
Interpretation:
The member that rapidly undergoes
Concept Introduction:
The rate of the reaction is depends on a single reactant in reaction is known as
The first step of
Frist step is the slow step also rate determining step so the rate of the reaction is depends on the concentration of substrate only.
Nucleophile attacks the both front and back side of carbocation in
Order of the substrate that favored in
(b)
Answer to Problem 9.25P
The member that rapidly undergoes
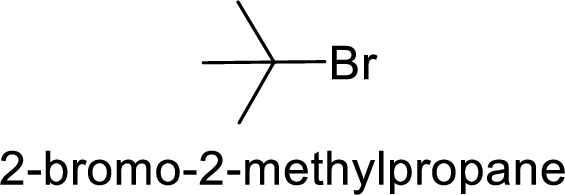
Explanation of Solution
Given:
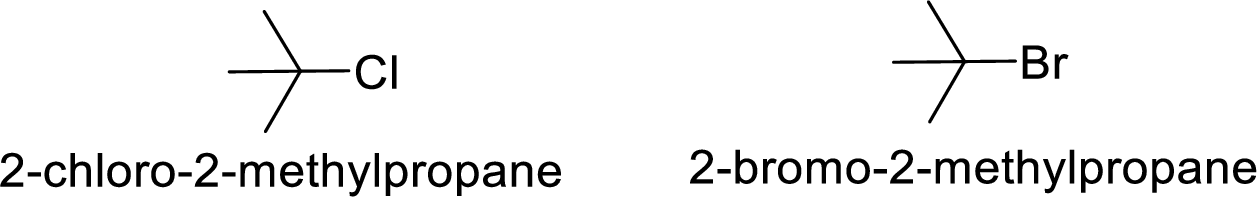
Bromine is better leaving group than chlorine.
Hence, the member that rapidly undergoes
(c)
Interpretation:
The member that rapidly undergoes
Concept Introduction:
The rate of the reaction is depends on a single reactant in reaction is known as
The first step of
Frist step is the slow step also rate determining step so the rate of the reaction is depends on the concentration of substrate only.
Nucleophile attacks the both front and back side of carbocation in
Order of the substrate that favored in
(c)
Answer to Problem 9.25P
The member that rapidly undergoes
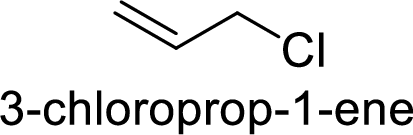
Explanation of Solution
Given:

In
Allyl cation is more stable than primary cation.
Hence, the member that rapidly undergoes
(d)
Interpretation:
The member that rapidly undergoes
Concept Introduction:
The rate of the reaction is depends on a single reactant in reaction is known as
The first step of
Frist step is the slow step also rate determining step so the rate of the reaction is depends on the concentration of substrate only.
Nucleophile attacks the both front and back side of carbocation in
Order of the substrate that favored in
(d)
Answer to Problem 9.25P
The member that rapidly undergoes
Explanation of Solution
Given:

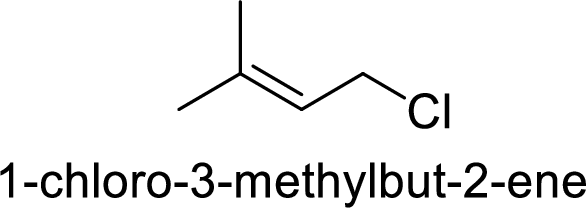
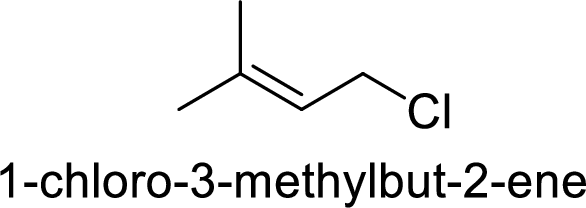
In
Substuted allyllic cation is more stable than allylic cation.
Hence, the member that rapidly undergoes
(e)
Interpretation:
The member that rapidly undergoes
Concept Introduction:
The rate of the reaction is depends on a single reactant in reaction is known as
The first step of
Frist step is the slow step also rate determining step so the rate of the reaction is depends on the concentration of substrate only.
Nucleophile attacks the both front and back side of carbocation in
Order of the substrate that favored in
(e)
Answer to Problem 9.25P
The member that rapidly undergoes
Explanation of Solution
Given:

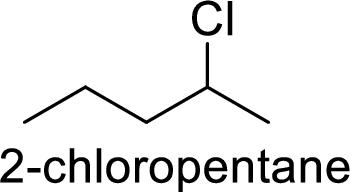
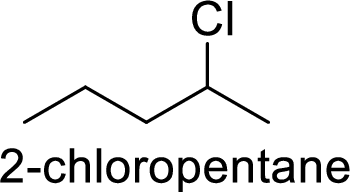
In
2-chloropentane gives more stable carbocation than 1-chloropentane.
Hence, the member that rapidly undergoes
(f)
Interpretation:
The member that rapidly undergoes
Concept Introduction:
The rate of the reaction is depends on a single reactant in reaction is known as
The first step of
Frist step is the slow step also rate determining step so the rate of the reaction is depends on the concentration of substrate only.
Nucleophile attacks the both front and back side of carbocation in
Order of the substrate that favored in
(f)
Answer to Problem 9.25P
The member that rapidly undergoes
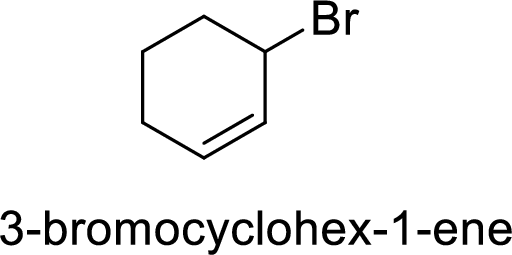
Explanation of Solution
Given:
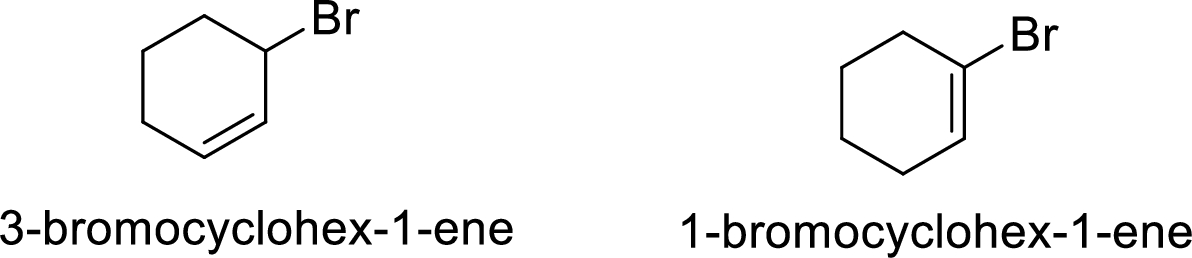
In
Allylic cation is more stable than vinyl cation.
Hence, the member that rapidly undergoes
Want to see more full solutions like this?
Chapter 9 Solutions
Organic Chemistry
- Definition and classification of boranes.arrow_forwardWhich of the terms explain the relationship between the two compounds? CH2OH Он Он Он Он α-D-galactose anomers enantiomers diastereomers epimers CH2OH ОН O он Он ОН B-D-galactosearrow_forwardHi, I need help on my practice final, If you could offer strategies and dumb it down for me with an explanation on how to solve that would be amazing and beneficial.arrow_forward
- Hi I need help with my practice final, it would be really helpful to offer strategies on how to solve it, dumb it down, and a detailed explanation on how to approach future similar problems like this. The devil is in the details and this would be extremely helpfularrow_forwardIn alpha-NbI4, Nb4+ should have the d1 configuration (bond with paired electrons: paramagnetic). Please comment.arrow_forwardHi, I need help on my practice final, if you could explain how to solve it offer strategies and dumb it down that would be amazing. Detail helpsarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


