
The exergy destruction associated with each of the processes of the Brayton cycle.
Answer to Problem 142P
The exergy destruction associated with process 1-2 of the given Brayton cycle is 28.08 kJ/kg.
The exergy destruction associated with process 2-3 of the given Brayton cycle is 100.3 kJ/kg.
The exergy destruction associated with process 3-4 of the given Brayton cycle is 32.86 kJ/kg.
The exergy destruction associated with process 4-1 of the given Brayton cycle is 197.93 kJ/kg.
Explanation of Solution
Show the simple Brayton cycle, and with air as the working fluid on T−s diagram.
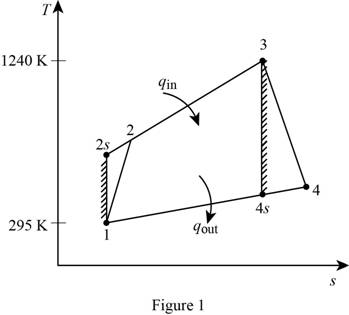
For the given simple Brayton cycle with air as the working fluid, let Ti, Pi, hi and Pri be the temperature, pressure, specific enthalpy and relative pressure at ith state respectively.
Write the expression of pressure ratio relation for the process 1-2.
Pr2=P2P1Pr1 (I)
Write the relation of pressure ratio and pressure for the process 3-4.
Pr4=P4P3Pr3 (II)
Write the expression of efficiency of the compressor (ηC).
ηC=h2s−h1h2−h1 (III)
Write the expression of efficiency of the turbine (ηT).
ηT=h3−h4h3−h4s (IV)
Write the expression of heat input to the Brayton cycle (qin).
qin=(h3−h2) (V)
Write the expression of heat rejected by the Brayton cycle (qout).
qout=(h4−h1) (VI)
Write the expression of exergy destruction associated with the process 1-2 of the given Brayton cycle (xdestroyed,12).
xdestroyed,12=T0(s2−s1)
xdestroyed,12=T0(s∘2−s∘1−RlnP2P1) (VII)
Here, temperature of the surroundings is T0, gas constant of air is R, entropy of air at state 2 as a function of temperature alone is s∘2, and entropy of air at state 1 as a function of temperature alone is s∘1.
Write the expression of exergy destruction associated with the process 2-3 of the given Brayton cycle (xdestroyed,23).
xdestroyed,23=T0(s3−s2+−qinTH)
xdestroyed,23=T0(s∘3−s∘2−RlnP3P2+−qinTH) (VIII)
Here, temperature of the heat source is TH, entropy of air at state 2 as a function of temperature alone is s∘2, and entropy of air at state 3 as a function of temperature alone is s∘3.
Write the expression of exergy destruction associated with the process 3-4 of the given Brayton cycle (xdestroyed,34).
xdestroyed,34=T0(s4−s3)
xdestroyed,34=T0(s∘4−s∘3−RlnP4P3) (IX)
Here, entropy of air at state 3 as a function of temperature is s∘3, and entropy of air at state 4 as a function of temperature is s∘4.
Write the expression of exergy destruction associated with the process 4-1 of the given Brayton cycle (xdestroyed,41).
xdestroyed,41=T0(s1−s4+qoutTL)
xdestroyed,41=T0(s∘1−s∘4−RlnP1P4+qoutTL) (X)
Here, temperature of the sink is TL, entropy of air at state 1 as a function of temperature alone is s∘1, and entropy of air at state 4 as a function of temperature alone is s∘4.
Conclusion:
Refer Table A-17E given in the textbook to find the properties of air at 295 K (T1), which gives 295.17 kJ/kg for h1, 1.3068 for Pr1 and 1.68515 kJ/kg⋅K for s∘1.
Substitute 10 for P2P1, and 1.3068 for Pr1 in Equation (I).
Pr2=(10)(1.3068)=13.07
Refer Table A-17E given in the textbook to find the properties of air at 13.07 (Pr1), which gives 570.26 kJ/kg for h2s, and 564.9 K for T2s.
Refer Table A-17E given in the textbook to find the properties of air at 1,240 K (T3), which gives 1,324.93 kJ/kg for h3, 272.3 for Pr3 and 3.21751 kJ/kg⋅K for s∘3.
Substitute 110 for P4P3, and 272.3 for Pr3 in Equation (II).
Pr4=(110)(272.3)=27.23
Refer Table A-17 given in the textbook to find the properties of air at 27.23 (Pr4), which gives 702.07kJ/kg for h4s, and 689.6 K for T4s.
Rearrange Equation (III) and substitute 295.17 kJ/kg for h1, 570.26 kJ/kg for h2s, and 0.83 for ηC.
h2=h1+h2s−h1ηC=295.17 kJ/kg+(570.26−295.17) kJ/kg0.83=626.60 kJ/kg
Refer Table A-17E given in the textbook to find the properties of air at 626.60 kJ/kg (h2), which gives 2.44117 kJ/kg⋅K for s∘2.
Rearrange Equation (IV) and substitute 1,324.93 kJ/kg for h3, 702.07 kJ/kg for h4s, and 0.87 for ηT.
h4=h3−ηT(h3−h4s)=1,324.93 kJ/kg+(0.87)(1,324.93−702.07) kJ/kg=783.04 kJ/kg
Refer Table A-17 given in the textbook, to find the properties of air at 783.04 kJ/kg (h4), which gives 764.4 K for T4 and 2.66807 kJ/kg⋅K for s∘4.
Substitute 1,324.93 kJ/kg for h3, and 626.60 kJ/kg for h2 in Equation (V).
qin=(1,324.93−626.60) kJ/kg=698.3 kJ/kg
Substitute 783.04 kJ/kg for h4, and 295.17 kJ/kg for h1 in Equation (VI).
qout=(783.04−295.17) kJ/kg=487.9 kJ/kg
Substitute 295 K for T0, 1.68515 kJ/kg⋅K for s∘1, 2.44117 kJ/kg⋅K for s∘2, 0.287 kJ/kg⋅K for R, and 10 for P2P1 in Equation (VII).
xdestroyed,12=(295 K)[(2.44117−1.68515 )kJ/kg⋅K−(0.287kJ/kg⋅K)ln(10)]=28.08 kJ/kg
Thus, the exergy destruction associated with process 1-2 of the given Brayton cycle is 28.08 kJ/kg.
Substitute 295 K for T0, 3.21751 kJ/kg⋅K for s∘3, 2.44117 kJ/kg⋅K for s∘2, 0.287 kJ/kg⋅K for R, 1,600 K for TH, and 698.3 kJ/kg for qin in Equation (VIII). For the process 2-3, pressure remains constant, hence substitute 0 for lnP3P2.
xdestroyed,23=(295 K)[(3.21751−2.44117 )kJ/kg⋅K−(0.287kJ/kg⋅K)(0)−698.3 kJ/kg1,600 K]=100.3 kJ/kg
Thus, the exergy destruction associated with process 2-3 of the given Brayton cycle is 100.3 kJ/kg.
Substitute 295 K for T0, 3.21751 kJ/kg⋅K for s∘3, 2.66807 kJ/kg⋅K for s∘4, 0.287 kJ/kg⋅K for R, and 110 for P4P3 in Equation (IX).
xdestroyed,34=(295 K)[(2.66807−3.21751 )kJ/kg⋅K−(0.287kJ/kg⋅K)ln(110)]=32.86 kJ/kg
Thus, the exergy destruction associated with process 3-4 of the given Brayton cycle is 32.86 kJ/kg.
Substitute 295 K for T0, 1.68515 kJ/kg⋅K for s∘1, 2.66807 kJ/kg⋅K for s∘4, 0.287 kJ/kg⋅K for R, 295 K for TL, and 487.9 kJ/kg for qout in Equation (X). For the process 4-1, pressure remains constant, hence substitute 0 for lnP1P4.
xdestroyed,41=(295 K)[(1.68515−2.66807)kJ/kg⋅K−(0.287kJ/kg⋅K)(0)+487.9 kJ/kg295 K]= 197.93 kJ/kg
Thus, the exergy destruction associated with process 4-1 of the given Brayton cycle is 197.93 kJ/kg.
Want to see more full solutions like this?
Chapter 9 Solutions
Thermodynamics: An Engineering Approach
- 2.5 (B). A steel rod of cross-sectional area 600 mm² and a coaxial copper tube of cross-sectional area 1000 mm² are firmly attached at their ends to form a compound bar. Determine the stress in the steel and in the copper when the temperature of the bar is raised by 80°C and an axial tensile force of 60 kN is applied. For steel, E = 200 GN/m² with x = 11 x 10-6 per °C. E = 100 GN/m² with α = 16.5 × 10-6 For copper, per °C. [E.I.E.] [94.6, 3.3 MN/m².]arrow_forward3–16. A particle of mass m is embedded at a distance R from the center of a massless circular disk of radius R which can roll without slipping on the inside surface of a fixed circular cylinder of radius 3R. The disk is released with zero velocity from the position shown and rolls because of gravity, all motion taking place in the same vertical plane. Find: (a) the maximum velocity of the particle during the resulting motion; (b) the reaction force acting on the disk at the point of contact when it is at its lowest position. KAR 60° 3R M Fig. P3-16arrow_forwardI have figured out the support reactions, Ay = 240 kN, Ax = 0 kN, Ma = 639.2 kN*m and the constant term for V(x) is 240. I am not figuring out the function of x part right. Show how to derive V(x) and M(x) for this distributed load.arrow_forward
- 2.4 (A). A 75 mm diameter compound bar is constructed by shrinking a circular brass bush onto the outside of a 50 mm diameter solid steel rod. If the compound bar is then subjected to an axial compressive load of 160 kN determine the load carried by the steel rod and the brass bush and the compressive stress set up in each material. For steel, E 210 GN/m²; for brass, E = 100 GN/m². [I. Struct. E.] [100.3, 59.7 kN; 51.1, 24.3 MN/m².]arrow_forward1.7 (A). A bar ABCD consists of three sections: AB is 25 mm square and 50 mm long, BC is of 20 mm diameter and 40 mm long and CD is of 12 mm diameter and 50 mm long. Determine the stress set up in each section of the bar when it is subjected to an axial tensile load of 20 kN. What will be the total extension of the bar under this load? For the bar material, E = 210GN/m2. [32,63.7, 176.8 MN/mZ, 0.062mrn.l 10:41 مarrow_forward2.2 (A). If the maximum stress allowed in the copper of the cable of problem 2.1 is 60 MN/m2, determine the maximum tension which C3.75 kN.1 10:41 مarrow_forward
- 1.1 (A). A 25mm squarecross-section bar of length 300mm carries an axial compressive load of 50kN. Determine the stress set up ip the bar and its change of length when the load is applied. For the bar material E = 200 GN/m2. [80 MN/m2; 0.12mm.larrow_forward2.1 (A). A power transmission cable consists of ten copper wires each of 1.6 mm diameter surrounding three steel wires each of 3 mm diameter. Determine the combined E for the compound cable and hence determine the extension of a 30 m length of the cable when it is being laid with a tension of 2 kN. For steel, E200 GN/mZ; for copper, E = 100 GN/mZ. C151.3 GN/mZ; 9.6 mm.] 10:41 مarrow_forwardquestion 662 thank youarrow_forward
- 1.5 (A). A simple turnbuckle arrangement is constructed from a 40 mm outside diameter tube threaded internally at each end to take two rods of 25 mm outside diameter with threaded ends. What will be the nominal stresses set up in the tube and the rods, ignoring thread depth, when the turnbuckle cames an axial load of 30 kN? Assuming a sufficient strength of thread, what maximum load can be transmitted by the turnbuckle if the maximum stress is limited to 180 MN/mz? C39.2, 61.1 MN/m2, 88.4 kN.1arrow_forward1.3 (A). Define the terms shear stress and shear strain, illustrating your answer by means of a simple sketch. Two circular bars, one of brass and the other of steel, are to be loaded by a shear load of 30 kN. Determine the necessary diameter of the bars (a) in single shear, (b) in double shear, if the shear stress in the two materials must not exceed 50 MN/m2 and 100 MN/ mZ respectively. C27.6, 19.5, 19.5, 13.8mm.l 11arrow_forward1.4 (A). Two forkend pieces are to be joined together by a single steel pin of 25mm diameter and they are required to transmit 50 kN. Determine the minimum cross-sectional area of material required in one branch of either fork if the stress in the fork material is not to exceed 180 MN/m2. What will be the maximum shear stress in the pin? C1.39 x 10e4mZ; 50.9MN/mZ.] 10:41arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





