
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
4th Edition
ISBN: 9781119776741
Author: Klein
Publisher: WILEY CONS
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 8.5, Problem 8ATS
Interpretation Introduction
Interpretation: The bicycle [3.1.0] hexane ring system is found in natural products like sabinene in black paper. The mechanism of the preparation of this molecule is to be interpreted from compound 1 to compound 3.
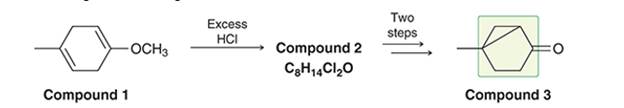
Concept Introduction:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Compound A has molecular formula C7H15B.. Treatment of compound A with sodium ethoxide yields only one elimination product
(compound B) and no substitution products. When compound B is treated with dilute sulfuric acid, compound C is obtained, which
has molecular formula C7H160. Draw the structures of compounds A, B, and C.
Compound A and compound B are in equilibrium. Write a stepwise mechanism from compound Ato compound B showing ALL intermediates. Use curved arrows to symbolize the flow of electrons to show how each of the intermediates and products are formed. Show all lone pairs and formal charges. Lastly, explain which compound (Aor B) will be in higher concentration.
The following questions concern ethyl (2-oxocyclohexane)carboxylate.(a) Write a chemical equation showing how you could prepare ethyl (2-oxocyclohexane)-carboxylate by a Dieckmann cyclization.(b) Write a chemical equation showing how you could prepare ethyl (2-oxocyclohexane)-carboxylate by acylation of a ketone.(c) Write structural formulas for the two most stable enol forms of ethyl (2-oxocyclohexane)carboxylate.(d) Write the three most stable resonance contributors to the most stable enolate derived from ethyl (2-oxocyclohexane)carboxylate.(e) Show how you could use ethyl (2-oxocyclohexane)carboxylate to prepare 2-methylcyclohexanone.(f) Give the structure of the product formed on treatment of ethyl (2-oxocyclohexane)-carboxylate with acrolein (H2C=CHCH=O) in ethanol in the presence of sodium ethoxide
Chapter 8 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
Ch. 8.3 - Provide a systematic name for each of the...Ch. 8.3 - Prob. 2CCCh. 8.3 - Prob. 3CCCh. 8.3 - Prob. 4CCCh. 8.5 - Prob. 5CCCh. 8.5 - Prob. 6CCCh. 8.5 - Prob. 1LTSCh. 8.5 - Prob. 7PTSCh. 8.5 - Prob. 8ATSCh. 8.5 - Prob. 9CC
Ch. 8.5 - Prob. 2LTSCh. 8.5 - Prob. 10PTSCh. 8.5 - Prob. 11ATSCh. 8.6 - Prob. 12CCCh. 8.6 - Prob. 13CCCh. 8.6 - Prob. 3LTSCh. 8.6 - Prob. 14PTSCh. 8.6 - Prob. 15ATSCh. 8.7 - Predict the product for each reaction, and predict...Ch. 8.7 - Prob. 17CCCh. 8.8 - Prob. 18CCCh. 8.8 - Prob. 19CCCh. 8.8 - Prob. 4LTSCh. 8.8 - Prob. 20PTSCh. 8.8 - Prob. 21ATSCh. 8.9 - Prob. 5LTSCh. 8.9 - Prob. 22PTSCh. 8.9 - Prob. 23ATSCh. 8.10 - Prob. 24CCCh. 8.10 - Prob. 6LTSCh. 8.10 - Prob. 25PTSCh. 8.10 - Prob. 26ATSCh. 8.10 - Prob. 27ATSCh. 8.11 - Prob. 7LTSCh. 8 - Prob. 47PP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Compound X is optically inactive and has the formula C 16H 16Br 2. On treatment with strong base, X gives hydrocarbon Y, C 16H 14. Compound Y absorbs 2 equivalents of hydrogen when reduced over a palladium catalyst and reacts with ozone to give two fragments. One fragment Z, is an aldehyde with formula C 7H 6O. The other fragment is glyoxal, (CHO)2. Which of the following answers is correct? Select all that are correct.arrow_forwardCompound X is optically inactive and has the formula C 16H 16Br 2. On treatment with strong base, X gives hydrocarbon Y, C 16H 14. Compound Y absorbs 2 equivalents of hydrogen when reduced over a palladium catalyst and reacts with ozone to give two fragments. One fragment Z, is an aldehyde with formula C 7H 6O. The other fragment is glyoxal, (CHO)2. Which of the following answers is correct? Select all that are correct.arrow_forward4) Aromatic compounds are among the most abundant and versatile in nature. From a synthetic point of view, these compounds, despite their stabilities, are quite useful and can undergo reactions under special conditions and by specific mechanisms, such as the Electrophilic Aromatic Substitution (SAE) and the Nucleophilic Aromatic Substitution (SNAr). Based on this, please answer the following items: (b) How would you prepare the following compounds starting from benzene? Explain the second in a different wayarrow_forward
- (c) Answer each of the questions below that relate to acetophenone: Xo (i) (ii) (iii) Draw the structure of the enol form of acetophenone. Give a stepwise mechanism for the conversion of acetophenone into its enol form. Show how each of the three compounds A, B and C below can be prepared from acetophenone. Explain clearly what reactants/reagents would be required in each case. odocor A B Br Carrow_forwardPropene reacts with hydrogen bromide to form two isomers, the major product being, 2- bromopropane. 1(c) Draw the mechanism of the reaction of hydrogen bromide with propene to form 1- bromopropane and 2-bromopropane, showing the structure of both intermediates. (i) (ii) Explain why 2-bromopropane is the major product.arrow_forwardUsing hex-1-ene as your starting material, show how you would synthesize the following compounds. (Once you haveshown how to synthesize a compound, you may use it as the starting material in any later parts of this problem.)(a) 1,2-dibromohexanearrow_forward
- Enanthotoxin is an extremely poisonous organic compound found in hemlock water drop- wart, which is reputed to be the most poisonous plant in England. It is believed that no British plant has been responsible for more fatal accidents. The most poisonous part of the plant is the roots, which resemble small white carrots, giving the plant the name"five finger death."Also poisonous are its leaves, which look like parsley. Enanthotoxin is thought to interfere with the Nat current in nerve cells, which leads to convulsions and death. он How many stereoisomers are possible for enanthotoxin?arrow_forwardConsider the tetracyclic aromatic compound drawn below, with rings labeled as A, B, C, and D. (a) Which of the four rings is most reactive in electrophilic aromatic substitution? (b) Which of the four rings is least reactive in electrophilic aromatic substitution? (c) What are the major product(s) formed when this compound is treated with one equivalent of Br2?arrow_forwardCompound A is an aromatic compound with the molecular formula C8H8. When treated with excess BR2, compound A is converted into compound B, with the molecular formula C8H8BR2. Draw the structure of compound a. arrow_forward
- Compound F may be synthesised by the method attached: When 2-chloropropane treated with NaOH and 1-chloropropane treated with NaOH separately produce two different functional groups. Provide both reactions and explain the two different functional groups produced.arrow_forwardCompound A undergoes an acid-catalyzed hydrolysis. One of the products (B) that is isolated gives the following 1H NMR spectrum. Identify the compounds A and Carrow_forward(b) Answer the following questions based on the compounds below. Jawab soalan berikut berdasarkan kepada sebatian di bawah. CI CI A в (i) Which compound has the higher boiling point? Explain. Sebatian manakah mempunyai takat didih yang lebih tinggi? Terangkan. (ii) Draw the SN2 mechanism for the reaction of compound A with sodium hydroxide, NaOH. Lukis mekanisma Sn2 bagi tindak balas antara sebatian A dengan natrium hidroksida, NaOH.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY