
Organic Chemistry - Standalone book
10th Edition
ISBN: 9780073511214
Author: Francis A Carey Dr., Robert M. Giuliano
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8, Problem 42P
The heats of reaction were measured for addition of
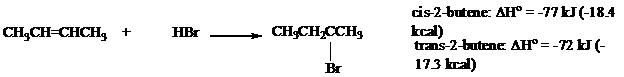
Use these data to calculate the energy difference between
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Compounds X and Y both have the formula C7H14.
Both X and Y react with one molar equivalent of hydrogen in the presence of a palladium catalyst to form 2-methylhexane.
The heat of hydrogenation of X is greater than that of Y.
Both X and Y react with HCI to give the same single C₂H₁5Cl compound as the major product.
What is the structure of X?
• In cases where there is more than one answer, just draw one.
7
0▾
ChemDoodleⓇ
146
21.) Calculate the enthalpy of hydrogenation of benzene to cyclohexane from the following
reactions
A,H (kJ/mol)
C6H6 (1) + 15/2 02 (g) → 6 CO2 (g) + 3 H20 (1)
C6H12 (1) + 9 02 (g) → 6 CO2 (g) + 6 H20 (1)
H2 (g) + ½ 02 (g) → H2O (1)
-3268
-3920
-285.83
a.) -205 kJ/mol
b.) -1507 kJ/mol
c.) -938 kJ/mol
d.) -366 kJ/mol
The rate law for addition of Br2 to an alkene is first order in Br2 and first order in the alkene. Does this information suggest that the mechanism of addition of Br2 to an alkene proceeds in the same manner as for addition of HBr? Explain.
Chapter 8 Solutions
Organic Chemistry - Standalone book
Ch. 8.1 - What three alkenes yield 2-methylbutane on...Ch. 8.2 - Prob. 2PCh. 8.2 - Prob. 3PCh. 8.3 - Prob. 4PCh. 8.4 - Prob. 5PCh. 8.4 - Give a structural formula for the carbocation...Ch. 8.5 - Prob. 7PCh. 8.6 - Instead of the three-step process of Mechanism...Ch. 8.6 - The rates of hydration of the two alkenes shown...Ch. 8.6 - Is the electrophilic addition of hydrogen chloride...
Ch. 8.7 - You can calculate the equilibrium constant for the...Ch. 8.7 - Does the presence or absence of a catalyst such as...Ch. 8.7 - The gas phase reaction of ethanol with hydrogen...Ch. 8.8 - Prob. 14PCh. 8.8 - Hydroborationoxidation of -pinene, like its...Ch. 8.10 - Arrange the compounds 2-methyl-1-butene,...Ch. 8.10 - Give the structure of the product formed when each...Ch. 8.11 - Prob. 18PCh. 8.11 - Prob. 19PCh. 8.12 - Prob. 20PCh. 8.12 - Prob. 21PCh. 8.13 - Prob. 22PCh. 8.14 - Prob. 23PCh. 8.14 - Prob. 24PCh. 8 - How many alkenes yield...Ch. 8 - Prob. 26PCh. 8 - Catalytic hydrogenation of...Ch. 8 - Prob. 28PCh. 8 - Prob. 29PCh. 8 - Prob. 30PCh. 8 - Prob. 31PCh. 8 - A single epoxide was isolated in 7984% yield in...Ch. 8 - Prob. 33PCh. 8 - Prob. 34PCh. 8 - On catalytic hydrogenation over a rhodium...Ch. 8 - Prob. 36PCh. 8 - Prob. 37PCh. 8 - Prob. 38PCh. 8 - Prob. 39PCh. 8 - 1-Butene has a higher heat of hydrogenation than...Ch. 8 - Match the following alkenes with the appropriate...Ch. 8 - The heats of reaction were measured for addition...Ch. 8 - Complete the following table by adding + and -...Ch. 8 - Match the heats of hydrogenation (107 kJ/mol,...Ch. 8 - The iodination of ethylene at 25 C is...Ch. 8 - Specify reagents suitable for converting...Ch. 8 - (a) Which primary alcohol of molecular formula...Ch. 8 - Identify compounds A and B in the retrosynthesis...Ch. 8 - Identify compounds A and B in the retrosynthesis...Ch. 8 - Prob. 50PCh. 8 - On being heated with a solution of sodium ethoxide...Ch. 8 - Compound A (C7H15Br) is not a primary alkyl...Ch. 8 - Prob. 53PCh. 8 - Prob. 54PCh. 8 - A mixture of three alkenes (A, B, and C) was...Ch. 8 - Reaction of 3,3-dimethyl-1-butene with hydrogen...Ch. 8 - Dehydration of 2,2,3,4,4-pentamethyl-3-pentanol...Ch. 8 - Prob. 58PCh. 8 - East Indian sandalwood oil contains a hydrocarbon...Ch. 8 - Prob. 60PCh. 8 - Prob. 61PCh. 8 - Prob. 62PCh. 8 - Prob. 63PCh. 8 - Prob. 64PCh. 8 - On the basis of the mechanism of acid-catalyzed...Ch. 8 - As a method for the preparation of alkenes, a...Ch. 8 - Which of the following is the most reasonable...Ch. 8 - Prob. 68PCh. 8 - Oxymercuration Concerns about mercurys toxicity...Ch. 8 - Prob. 70DSPCh. 8 - Prob. 71DSPCh. 8 - Prob. 72DSPCh. 8 - Prob. 73DSPCh. 8 - Oxymercuration Concerns about mercurys toxicity...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The rate law for addition of Br2 to an alkenes is first order in Br2 and first order in the alkene. Does this information suggest that the mechanism of addition of Br2 to an alkene proceeds in the same matter as for addition of HBr? Explain.arrow_forwardAn alkene forms 2-methylpropane when catalytically reduced and 1,2-dichloro-2-methylpropane when treated with Cl₂. Draw the structural formula of the alkene. • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. / √ [F ? ChemDoodlearrow_forwardCompounds X and Y both have the formula C7H₁4. Both X and Y react with one molar equivalent of hydrogen in the presence of a palladium catalyst to form 2-methylhexane. The heat of hydrogenation of X is greater than that of Y. Both X and Y react with HCI to give the same single C7H15Cl compound as the major product. What is the structure of X? • In cases where there is more than one answer, just draw one. 23 ▾ Sn [F ChemDoodleⓇ 146arrow_forward
- All about Alkene, Alkyne and Alkyl halides(1) Write a complete chemical equation showing reactants, products, and catalysts needed (if any) for the following reaction and (2) Draw and name the organic compound found in every reaction.(a) Complete hydrogenation of 2-Methylhexa-1,5-diene (b) Complete halogenation (Br2) of 3-Ethyl-2,2-dimethylhept-3-ene(c) Reaction of (4E)-2,4-Dimethylhexa-1,4-diene with a mole of waterarrow_forwardwhat are the physical porpeties of 1,3,5-cyclohexene thank youarrow_forwardDraw the structure of 2,3-dimethyl-1-butene. • Show stereochemistry only if given in the name. • You do not have to explicitly draw H atoms.arrow_forward
- The heat of combustion of decahydronaphthalene (C10H18) is -6286 kJ/mol. The heat of combustion of naphthalene 1C10H82 is -5157 kJ/mol. (In both cases CO2(g) and H2O(l) are the products). Calculate the heat of hydrogenation and the resonance energy of naphthalene.arrow_forwardConsider the following proposed structures for benzene, each of which is consistent with the molecular formula C6H6. (iv) CH3CCCCCH3 (v) CH2=CHCCH=CH2 When benzene reacts with chlorine to give C6H5Cl, only one isomer of that compound forms. Which of the five proposed structures for benzene are consistent with this observation? When C6H5Cl reacts further with chlorine to give C6H4Cl2, exactly three isomers of the latter compound form. Which of the five proposed structures for benzene are consistent with this observation?arrow_forward9) For the reaction between isopropyl 1-propyl (or 'n-propyl') ether and HBr, what are the expected main products? A) one alcohol and one alkyl bromide B) one alcohol and one alkene C) two alcohols D) one alcohol and one alkanearrow_forward
- Compound A is unsaturated hydrocarbon with molecular formula (C6H12) reacted with Br2 in water to form compound B. compound C was produced from the reaction between compound A, sulphuric acid and H2O (g). Compound A undergo hydrogenation to form compound D. Compound E was produced from the reaction of compound A with Br2 in room temperature. Compound A undergo hydrohalogenation in the presences of hydrogen peroxide to form compound F. The reaction between compound F with aqueous sodium hydroxide will form compound G. Compound H was produced when compound F reacts with the aqueous ammonia in ethanol. Compound F also reacts with aqueous sodium cyanide to produce compound I. Draw the possible structural formulae of compounds A, B, C, D, E, F, G, H and I. Give the IUPAC nomenclature of compounds H and I. Distinguish between compound A and D.arrow_forwardCompounds Y and Z both have the formula C₂H18. Both Y and Z react with one molar equivalent of hydrogen in the presence of a palladium catalyst to form 2-methyloctane. The heat of hydrogenation of Y is less than that of Z. Y and Z each undergo hydroboration/oxidation to give a primary alcohol (OH attached to a primary carbon). What is the structure of Y? • In cases where there is more than one answer, just draw one. 1998) 0▾ + n [F ChemDoodle aarrow_forwardAn unknown hydrocarbon A with the formula C6H12 reacts with 1 molar equivalent of H2 over a palladium catalyst. Hydrocarbon A also reacts with OsO4 to give diol B. When oxidized with KMnO4 in acidic solution, A gives two fragments. One fragment is propanoic acid, CH3CH2CO2H, and the other fragment is ketone C. What are the structures of A, B, and C? Write all reactions, and show your reasoning.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License