
Concept explainers
Oxymercuration
Concerns about mercury’s toxicity have led to decreased use of mercury-based reagents in
Among the synthetically useful reactions of
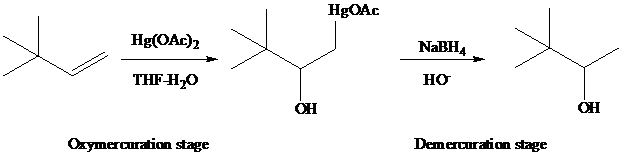
The reaction is performed in two operations, the first of which is oxymercuration. In this stage the alkene is treated with
From the overall reaction, we see that oxymercuration–demercuration
1. accomplishes hydration of the double bond in accordance with Markovnikov’s rule, and
2. carbocation rearrangements do not occur.
Additional information from stereochemical studies with other
3. anti addition of
4. the replacement of
The structure of the intermediate in oxymercuration has received much attention and can beapproached by considering what is likely to happen when the electrophile
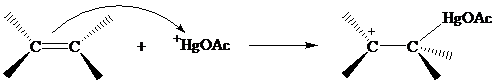
Recall from Section
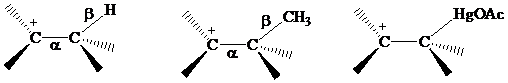
The electrons in a
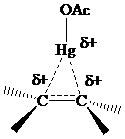
The problems that follow explore various synthetic aspects of oxymercuration–demercuration.Experimental procedures sometimes vary depending on the particular transformation. The source of the electrophile may be a mercury(II) salt other than
Oxymercuration–demercuration of
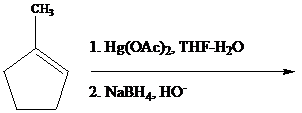
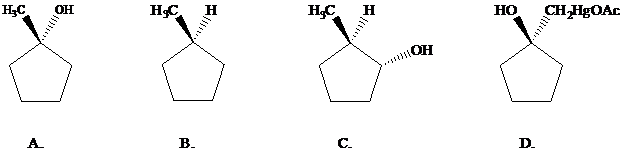
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Organic Chemistry - Standalone book
- A problem often encountered in the oxidation of primary alcohols to acids is that esters are sometimes produced as by-products. For example, oxidation of ethanol yields acetic acid and ethyl acetate: Propose a mechanism to account for the formation of ethyl acetate. Take into account the reversible reaction between aldehydes and alcohols:arrow_forwardChemistry Provide reagents/conditions to accomplish the following syntheses. Several steps are required in some cases.arrow_forwardA task is assigned to an undergraduate student to test two samples (known as compounds K and L) in the laboratory. She placed these two compounds through various scientific tests. She discovered that these compounds have the same molecular formula, CSHSO. When treated with 2,4-dinitrophenylhydrazine, all of these compounds produce brightly coloured precipitate, and both are reduced to an organic compound with the molecular formula C§H100. However, compound K can be easily oxidized by chromic acid to formed compound N and vice versa for compound L. Furthermore, when both compounds react with Fehling's solutions, they produce negative results. However, only compound K forms a silver mirror when it reacts with Tollen's reagent, and compound L does not. Identify the possible structural formulae for compounds K, L, and N by ignoring their position isomerism. Indicate the formation of compound N from compound K. Predict the chemical reaction that occurs when compound L reacts with 2,4-…arrow_forward
- I need a detailed diagram or table of every reaction in organic of: alcohol, aldehyde, ketone, epoxide, ester, ether, amide, anhydride, carboxylic acid, and functional derivatives of carboxylic acid. How to go from one to another, and the elaborated cycle of the reactionsarrow_forwardWhat are the various synthesis of linear, branched, and/or cyclic ethers? Show reaction schemes, discuss reaction mechanisms step by step, and indicate any limitations or advantages of the approach.arrow_forwardSynthesize the following compounds starting from benzene. Reactions contain more than two steps. Assume that you can separate ortho and para isomers if necessaryarrow_forward
- Provide the principle organic product(s) for the following reactions. If more than one product is expected, indicate the major product. Indicate stereochemistry where appropriate.arrow_forwardA) Provide the reagent and reaction mechanism to show how the reactants and products in the following reaction can interconvert B) under what conditions would the reaction I) favour reactants, II) favour the products and III) why?arrow_forward3) A common practice that organic chemists work on is a process known as retrosynthesis. In this a chemist will take known reactants and devise a synthetic pathway to produce the desired products using reactions that they know. As you proceed through organic chemistry you will be tasked with thinking about this process. How would you make the desired final product starting from the alkyne. It will take a couple of reactions. Please show the reagents necessary. And for your last step please show the arrow pushing. Starting material Br Product Br ...arrow_forward
- Grignard reagent is a versatile tool in synthetic organic chemistry. Using bromocyclopentane as a starting material, show how a Grignard reagent, X, is synthesized. Reaction of X with water produces compound Y while treatment in carbon dioxide followed by hydrolysis forms compound Z. 3-methyl-2butanone reacts with X and hydrolyses to yield compound AA. Draw the structural formulae of compounds Y, Z and AA and write the chemical equations respectively.arrow_forwardIndustrial Chemistry, Benzene is a high-commodity aromatic material with many industrial applications but is produced in relatively small amounts through petroleum fractionation. Explain how Toluene can be converted to benzene, please be specific in terms of reagents and conditions.arrow_forwardConsider the monosubstituted benzene reagent X. Nitration of X yields product Y. Product Y is analysed by 1H and 13C NMR spectroscopy (see Figures 1 to 4). Determine the structure of X and Y on the basis of the spectral data provided.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

