
Concept explainers
Interpretation:
The line graph of given mass and volume data needs to be drawn to interpret the slope of line. Also, the information from that slope of line needs to be deduced.
| Volume | Mass |
| 4.1 mL | 9.36 g |
| 6.0 mL | 14.04 g |
| 8.0 mL | 18.72 g |
| 10.0 mL | 23.40 g |
Concept introduction:
Both mass and volume are physical quantity which are related to each other. The mass of substance per volume is called as density of that substance.
Answer to Problem 138A
Slope = 2.73 g/mL
Slope of line curve gives the density of substance.
Explanation of Solution
Given information:
Mass and volume have linear relation.
Plot the curve with the given data:
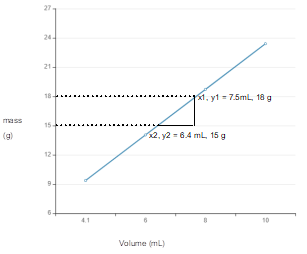
Calculate slope:
The slope of line is mass per volume that is density of substance.
Slope of line curve from mass and volume data gives the density of substance.
Chapter 8 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Essential Organic Chemistry (3rd Edition)
Inorganic Chemistry
General, Organic, and Biological Chemistry (3rd Edition)
CHEMISTRY-TEXT
Organic Chemistry (8th Edition)
Chemistry: A Molecular Approach (4th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





