
(a)
Interpretation:
The stereoisomer products for the given reaction should be determined.
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Electrophilic addition: It is a type of addition reaction in which the pi bond present in the molecule breaks as the electrophile approaches and results in the formation of product with sigma bond.
In addition reaction of
Oxidation Reaction: It involves loss of electrons, addition of oxygen atoms or removal of hydrogen atoms.
E configuration: The geometric isomers are given E configuration if high priority groups are placed on opposite sides of the bond.
Z configuration: The geometric isomers are given Z configuration if high priority groups are placed on same sides of the bond.
Stereo specific: The reaction is considered as stereo specific if the reactant is stereo isomers that give rise to different set of stereo isomers.
Stereoisomers: Two compounds with same molecular formula but different in their orientation are considered as isomers.
The presence of atom with non-super impossible mirror image is defined as enantiomers which are given
(b)
Interpretation:
The stereoisomer products for the given reaction should be determined.
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Electrophilic addition: It is a type of addition reaction in which the pi bond present in the molecule breaks as the electrophile approaches and results in the formation of product with sigma bond.
In addition reaction of alkenes when two substituents approaches same side of
Oxidation Reaction: It involves loss of electrons, addition of oxygen atoms or removal of hydrogen atoms.
E configuration: The geometric isomers are given E configuration if high priority groups are placed on opposite sides of the bond.
Z configuration: The geometric isomers are given Z configuration if high priority groups are placed on same sides of the bond.
Stereo specific: The reaction is considered as stereo specific if the reactant is stereo isomers that give rise to different set of stereo isomers.
Stereoisomers: Two compounds with same molecular formula but different in their orientation are considered as isomers.
The presence of atom with non-super impossible mirror image is defined as enantiomers which are given
(c)
Interpretation:
The stereoisomer products for the given reaction should be determined.
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Electrophilic addition: It is a type of addition reaction in which the pi bond present in the molecule breaks as the electrophile approaches and results in the formation of product with sigma bond.
Oxidation Reaction: It involves loss of electrons, addition of oxygen atoms or removal of hydrogen atoms.
Acid Catalyzed Hydration Reaction: The reaction involves breaking of phi bonds between carbon-carbon multiple bonds and addition of alcohol to more substituted position of carbon in the molecule.
First step is the acid donates proton to the alkene which leads to the formation of more stable carbo cation.
Then, the water is added to the given alkene through acid catalyzed reaction where the water gets added to the carbo cation finally, the removal of one proton from oxonium ion (oxygen with one positive charge) using water results in the formation of product.
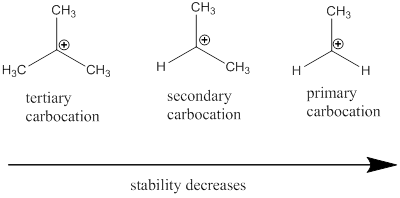
Carbocation: it is carbon ion that bears a positive charge on it.
Carbocation stability order:
(d)
Interpretation:
The stereoisomer products for the given reaction should be determined.
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Electrophilic addition: It is a type of addition reaction in which the pi bond present in the molecule breaks as the electrophile approaches and results in the formation of product with sigma bond.
In addition reaction of alkenes when two substituents approaches same side of
Oxidation Reaction: It involves loss of electrons, addition of oxygen atoms or removal of hydrogen atoms.
E configuration: The geometric isomers are given E configuration if high priority groups are placed on opposite sides of the bond.
Z configuration: The geometric isomers are given Z configuration if high priority groups are placed on same sides of the bond.
Stereo specific: The reaction is considered as stereo specific if the reactant is stereo isomers that give rise to different set of stereo isomers.
Stereoisomers: Two compounds with same molecular formula but different in their orientation are considered as isomers.
The presence of atom with non-super impossible mirror image is defined as enantiomers which are given
(e)
Interpretation:
The stereoisomer products for the given reaction should be determined.
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Electrophilic addition: It is a type of addition reaction in which the pi bond present in the molecule breaks as the electrophile approaches and results in the formation of product with sigma bond.
In addition reaction of alkenes when two substituents approaches same side of
Oxidation Reaction: It involves loss of electrons, addition of oxygen atoms or removal of hydrogen atoms.
E configuration: The geometric isomers are given E configuration if high priority groups are placed on opposite sides of the bond.
Z configuration: The geometric isomers are given Z configuration if high priority groups are placed on same sides of the bond.
Stereo specific: The reaction is considered as stereo specific if the reactant is stereo isomers that give rise to different set of stereo isomers.
Stereoisomers: Two compounds with same molecular formula but different in their orientation are considered as isomers.
The presence of atom with non-super impossible mirror image is defined as enantiomers which are given
(f)
Interpretation:
The stereoisomer products for the given reaction should be determined.
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Electrophilic addition: It is a type of addition reaction in which the pi bond present in the molecule breaks as the electrophile approaches and results in the formation of product with sigma bond.
In addition reaction of alkenes when two substituents approaches same side of
Oxidation Reaction: It involves loss of electrons, addition of oxygen atoms or removal of hydrogen atoms.
E configuration: The geometric isomers are given E configuration if high priority groups are placed on opposite sides of the bond.
Z configuration: The geometric isomers are given Z configuration if high priority groups are placed on same sides of the bond.
Stereo specific: The reaction is considered as stereo specific if the reactant is stereo isomers that give rise to different set of stereo isomers.
Stereoisomers: Two compounds with same molecular formula but different in their orientation are considered as isomers.
The presence of atom with non-super impossible mirror image is defined as enantiomers which are given
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Organic Chemistry (8th Edition)
- Indicate the correct option.a) Graphite conducts electricity, being an isotropic materialb) Graphite is not a conductor of electricityc) Both are falsearrow_forward(f) SO: Best Lewis Structure 3 e group geometry:_ shape/molecular geometry:, (g) CF2CF2 Best Lewis Structure polarity: e group arrangement:_ shape/molecular geometry: (h) (NH4)2SO4 Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forward1. Problem Set 3b Chem 141 For each of the following compounds draw the BEST Lewis Structure then sketch the molecule (showing bond angles). Identify (i) electron group geometry (ii) shape around EACH central atom (iii) whether the molecule is polar or non-polar (iv) (a) SeF4 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: (b) AsOBr3 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles):arrow_forward
- (c) SOCI Best Lewis Structure 2 e group arrangement: shape/molecular geometry:_ (d) PCls Best Lewis Structure polarity: e group geometry:_ shape/molecular geometry:_ (e) Ba(BrO2): Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forwardDon't used Ai solutionarrow_forwardDon't used Ai solutionarrow_forward
- reaction scheme for C39H4202 Hydrogenation of Alkyne (Alkyne to Alkene) show reaction (drawing) pleasearrow_forwardGive detailed mechanism Solution with explanation needed. Don't give Ai generated solutionarrow_forwardShow work with explanation needed....don't give Ai generated solutionarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





