
Concept explainers
(a)
Interpretation:
The
(b)
Interpretation:
The name and
(c)
Interpretation:
The number of neutrons present in the element has to be given.
(d)
Interpretation:
The charge on the ion and the type of ion has to be identified.
(e)
Interpretation:
The symbolic notation of the element has to be written.
Concept Introduction:
Mass number is the sum of the protons and neutrons in the nucleus.
Atomic number is the number of protons present in the nucleus.
For an element
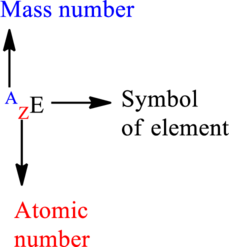
The letter A represents mass number.
The letter Z represents atomic number.
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
EBK FOUNDATIONS OF COLLEGE CHEMISTRY
- From the following written description, write the balanced chemical equation for the reaction including state symbols. A diatomic gaseous molecule that contains 17 protons per atom is reacted with a solid element that has an atomic number of 19 to yield an ionic compound.arrow_forwardGive the complete symbol (XZA), including atomic number and mass number, of (a) a nickel atom with 31 neutrons, and (b) a tungsten atom with 110 neutrons.arrow_forwardGive the complete symbol(ZAX), including atomic number and mass number, of (a) a nickel atom with 31 neutrons, (b) a plutonium atom with 150 neutrons, and (c) a tungsten atom with 110 neutrons.arrow_forward
- The formula of water is If-O. Which of the following is indicated by this formula? Explain your answer. a. The mass of hydrogen is twice that of oxygen in each molecule. b. There are two hydrogen atoms and one oxygen atom per water molecule. c. The mass of oxygen is twice that of hydrogen in each molecule. d. There are two oxygen atoms and one hydrogen atom per water molecule.arrow_forwardIn a hypothetical universe, an oil-drop experiment gave the following measurements of charges on oil drops: 5.55 1019 C, 9.25 1019 C, 1.11 1018 C, and 1.48 1018 C. Assume that the smallest difference in charge equals the unit of negative charge in this universe. What is the value of this unit of charge? How many units of excess negative charge are there on each oil drop?arrow_forwardUsing the information in Table 2.1, answer the following questions. In an ion with an unknown charge, the total mass of all the electrons was determined to be 2.55 1026 g. while the total mass of its protons was 5.34 1023 g. What is the identity and charge of this ion? What is the symbol and mass number of a neutral atom whose total mass of its electrons is 3.92 1026 g, while its neutrons have a mass of 9.35 1023 g?arrow_forward
- 2.90 Naturally occurring europium has an average atomic weight of 151.964 amu. If the only isotopes of europium present are 151Eu and 153Eu, describe how you would determine the relative abundance of the two isotopes. Include in your description any information that would need to be looked up.arrow_forwardThe photo here depicts what happens when a coil of magnesium ribbon and a few calcium chips are placed in water. (a) Based on these observations, what might you expect to see when barium, another Croup 2A element, is placed in water? (b) Give the period in which each element (Mg. Ca, and Ba) is found. What correlation do you think you might find between the reactivity of these elements and their positions in the periodic table?arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





