
EBK FOUNDATIONS OF COLLEGE CHEMISTRY
15th Edition
ISBN: 9781118930144
Author: Willard
Publisher: JOHN WILEY+SONS INC.
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 5, Problem 20PE
(a)
Interpretation Introduction
Interpretation:
The isotopic notation symbol for
Concept Introduction:
Mass number is the sum of the protons and neutrons in the nucleus.
For an element
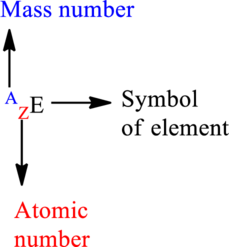
The letter A represents mass number.
The letter Z represents atomic number.
(b)
Interpretation Introduction
Interpretation:
The isotopic notation symbol for
Concept Introduction:
Refer to part (a).
(c)
Interpretation Introduction
Interpretation:
The isotopic notation symbol for
Concept Introduction:
Refer to part (a).
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
а 1 (а)
234
Uranium has three common isotopes. If the abundance of
U is 0.01%, the
235
´U is 0.71%, and the abundance of
238
abundance of
´U is 99.28%, what is the
average atomic mass of uranium?
Q
1(b)
A graduated cylinder has a mass of 80g when empty. When 20mL of water is added,
the graduated cylinder has a mass of 100g. If a stone is added to the graduated
cylinder, the water level rises to 45mL and the total mass is 156g. What is the
density of the stone in g/cm3?
Q 1 (с)
How many grams are there in 985mL of nitrogen (N2) at 0.0°C and 1.00×10°mmHg?
Identify each of the following elements as a metal, nonmetal, or metalloid: (a) phosphorus, (b) strontium?
Chapter 5 Solutions
EBK FOUNDATIONS OF COLLEGE CHEMISTRY
Ch. 5.1 - Prob. 5.1PCh. 5.2 - Prob. 5.2PCh. 5.3 - Prob. 5.3PCh. 5.4 - Prob. 5.4PCh. 5.5 - Prob. 5.5PCh. 5.5 - Prob. 5.6PCh. 5.6 - Prob. 5.7PCh. 5 - Prob. 1RQCh. 5 - Prob. 2RQCh. 5 - Prob. 3RQ
Ch. 5 - Prob. 4RQCh. 5 - Prob. 5RQCh. 5 - Prob. 6RQCh. 5 - Prob. 7RQCh. 5 - Prob. 8RQCh. 5 - Prob. 9RQCh. 5 - Prob. 10RQCh. 5 - Prob. 11RQCh. 5 - Prob. 12RQCh. 5 - Prob. 1PECh. 5 - Prob. 2PECh. 5 - Prob. 3PECh. 5 - Prob. 4PECh. 5 - Prob. 5PECh. 5 - Prob. 6PECh. 5 - Prob. 7PECh. 5 - Prob. 8PECh. 5 - Prob. 9PECh. 5 - Prob. 10PECh. 5 - Prob. 11PECh. 5 - Prob. 12PECh. 5 - Prob. 13PECh. 5 - Prob. 14PECh. 5 - Prob. 15PECh. 5 - Prob. 16PECh. 5 - Prob. 17PECh. 5 - Prob. 18PECh. 5 - Prob. 19PECh. 5 - Prob. 20PECh. 5 - Prob. 21PECh. 5 - Prob. 22PECh. 5 - Prob. 23PECh. 5 - Prob. 24PECh. 5 - Prob. 25PECh. 5 - Prob. 26PECh. 5 - Prob. 27PECh. 5 - Prob. 28PECh. 5 - Prob. 29PECh. 5 - Prob. 30PECh. 5 - Prob. 31PECh. 5 - Prob. 32PECh. 5 - Prob. 33PECh. 5 - Prob. 34PECh. 5 - Prob. 35AECh. 5 - Prob. 36AECh. 5 - Prob. 37AECh. 5 - Prob. 38AECh. 5 - Prob. 39AECh. 5 - Prob. 42AECh. 5 - Prob. 43AECh. 5 - Prob. 45AECh. 5 - Prob. 46AECh. 5 - Prob. 47AECh. 5 - Prob. 48AECh. 5 - Prob. 49AECh. 5 - Prob. 50AECh. 5 - Prob. 51AECh. 5 - Prob. 53AECh. 5 - Prob. 54AECh. 5 - Prob. 55AECh. 5 - Prob. 56AECh. 5 - Prob. 60CE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In what way are isotopes of a given element always different? In what way(s) are they always the same?arrow_forward2-69 (Chemical Connections 2A) Why does the body need sulfur, calcium, and iron?arrow_forward2-99 A 7.12 g sample of magnesium is heated with 1.80 g of bromine. All the bromine is used up, and 2.07 g of magnesium bromide is produced. What mass of magnesium remains unreacted?arrow_forward
- 2-35 The two most abundant naturally occurring isotopes of carbon are carbon-12 (98.90%, 12.000 amu) and carbon-13 (1.10%, 13.003 amu). From these abundances, calculate the atomic weight of carbon and compare your calculated value with that given in the Periodic Table.arrow_forward2-85 The mass of a proton is 1.67 × 10-24g. The mass of a grain of salt is 1.0 × 10-2g. How many protons would it take to have the same mass as a grain of salt?arrow_forwardHow do isotopes of a given element differ? How are they similar?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning