
Concept explainers
(a)
Interpretation:
The number of protons, neutrons and electrons in
Concept Introduction:
Mass number is the sum of the protons and neutrons in the nucleus.
For an element
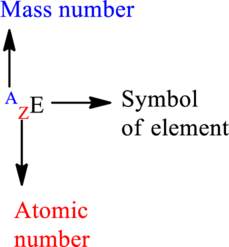
The letter A represents mass number.
The letter Z represents atomic number.
(a)
Answer to Problem 22PE
The number of protons in
The number of electrons in
The number of neutrons in
Explanation of Solution
The atomic number of
The mass number of
Atomic number of an element tells about the number of protons present.
The number of protons present will be equal to the number of electrons.
Atomic number of
The number of neutrons is the difference between the mass number and the atomic number.
The number of neutrons is calculated as,
Number of neutrons=
Number of neutrons=
The number of protons in
The number of electrons in
The number of neutrons in
(b)
Interpretation:
The number of protons, neutrons and electrons in
Concept Introduction:
Refer to part (a).
(b)
Answer to Problem 22PE
The number of protons in
The number of electrons in
The number of neutrons in
Explanation of Solution
The atomic number of
The mass number of
Atomic number of an element tells about the number of protons present.
The number of protons present will be equal to the number of electrons.
Atomic number of
The number of neutrons is the difference between the mass number and the atomic number.
The number of neutrons is calculated as,
Number of neutrons=
Number of neutrons=
The number of protons in
The number of electrons in
The number of neutrons in
(c)
Interpretation:
The number of protons, neutrons and electrons in
Concept Introduction:
Refer to part (a).
(c)
Answer to Problem 22PE
The number of protons in
The number of electrons in
The number of neutrons in
Explanation of Solution
The atomic number of
The mass number of
Atomic number of an element tells about the number of protons present.
The number of protons present will be equal to the number of electrons.
Atomic number of
The number of neutrons is the difference between the mass number and the atomic number.
The number of neutrons is calculated as,
Number of neutrons=
Number of neutrons=
The number of protons in
The number of electrons in
The number of neutrons in
(d)
Interpretation:
The number of protons, neutrons and electrons in
Concept Introduction:
Refer to part (a).
(d)
Answer to Problem 22PE
The number of protons in
The number of electrons in
The number of neutrons in
Explanation of Solution
The atomic number of
The mass number of
Atomic number of an element tells about the number of protons present.
The number of protons present will be equal to the number of electrons.
Atomic number of
The number of neutrons is the difference between the mass number and the atomic number.
The number of neutrons is calculated as,
Number of neutrons=
Number of neutrons=
The number of protons in
The number of electrons in
The number of neutrons in
Want to see more full solutions like this?
Chapter 5 Solutions
EBK FOUNDATIONS OF COLLEGE CHEMISTRY
- Define the term atomic weight. Why might the values of atomic weights on a planet elsewhere in the universe be different from those on earth?arrow_forwardThere are 2.619 1022 atoms in 1.000 g of sodium. Assume that sodium atoms are spheres of radius 1.86 and that they are lined up side by side. How many miles in length is the line of sodium atoms?arrow_forward2-69 (Chemical Connections 2A) Why does the body need sulfur, calcium, and iron?arrow_forward
- If the volume of a proton is similar to the volume of an electron, how will the densities of these two particles compare to each other?arrow_forwardThe following table presents the abundances and masses of the isotopes of zinc. What is the atomic weight of zinc?arrow_forward2.19 Naturally occurring uranium consists of two isotopes, whose masses and abundances are shown below: Only 235U can be used as fuel in a nuclear reactor, so uramium for use in the nuclear industry must be enriched in this isotope. If a sample of enriched uranium has an atomic weight of 235.684 amu, what percentage of 235LT is present?arrow_forward
- Two elements, R and Q, combine to form two binary compounds. In the first compound, 14.0 g of R combines with 3.00 g of Q. In the second compound, 7.00 g of R combines with 4.50 g of Q. Show that these data are in accord with the law of multiple proportions. If the formula of the second compound is RQ, what is the formula of the first compound?arrow_forwardGive the complete symbol (XZA), including atomic number and mass number, of (a) a nickel atom with 31 neutrons, and (b) a tungsten atom with 110 neutrons.arrow_forward2.90 Naturally occurring europium has an average atomic weight of 151.964 amu. If the only isotopes of europium present are 151Eu and 153Eu, describe how you would determine the relative abundance of the two isotopes. Include in your description any information that would need to be looked up.arrow_forward
- Which of the following are isotopes of element X, the atomic number for which is 9: 919X, 920X, 189X, and 921X?arrow_forwardOxygen consists of three different _____, each having eight protons but different numbers of neutrons.arrow_forwardGive the complete symbol(ZAX), including atomic number and mass number, of (a) a nickel atom with 31 neutrons, (b) a plutonium atom with 150 neutrons, and (c) a tungsten atom with 110 neutrons.arrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





