
Interpretation:
The given molecular art should be identified as
Concept introduction:
Chemical reaction is process when one or more substances get converted to different products. These substances can be elements or compounds. In chemical reaction, the atoms of the reactants break down and react with the other individual elements to form new products. Through this process there is change in the identity of the substance.
Physical change is brought about by changing the physical nature of the substance without changing the composition.
Answer to Problem 23P
From the art pictorial representation, it is said to be a chemical change.
Explanation of Solution
Given:
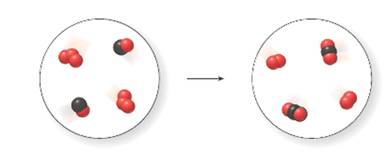
For the given molecular art, let the red balls represent oxygen atom and black balls represents carbon atoms.
Thus, in the reactant side of the molecular art, there are 3 atoms of O combine together forming ozone, O3 and one atom of O combine with one atom of C forming carbon monoxide, CO whereas in the product side of the molecular art, there are two atoms of O combined together to form dioxygen, O2 and two atoms of O combine with one atom of C forming carbon dioxide, CO2.
The equation is written as:
O3+CO→O2+CO2
Since, no two substances formed at product side are similar to the initial substance on the reactant side. So, the change is chemical.
The reaction is balanced as the number of atoms of each element on the reactant side and product side are equal.
The picture depiction indicates that it is a chemical change as there is changes in the identity and alteration of the composition.
Want to see more full solutions like this?
Chapter 5 Solutions
General, Organic, and Biological Chemistry - 4th edition
- Please see photoarrow_forward=Naming benzene derivatives Name these organic compounds: structure C1 CH3 name ☐ CH3 ப C1 × ☐arrow_forwardBlocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see image **NOTE: The compound on the left is the starting point, and the compound on the right is the final product. Please show the steps in between to get from start to final, please. These are not two different compounds that need to be worked.arrow_forward
- Nucleophilic Aromatic Substitution: What is the product of the reaction? What is the name of the intermediate complex? *See imagearrow_forwardPredict the final product. If 2 products are made, list which should be “major” and “minor” *see attachedarrow_forwardNucleophilic Aromatic Substitution: What is the product of the reaction? *see imagearrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div





